Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy
- PMID: 15501933
- PMCID: PMC1304998
- DOI: 10.1529/biophysj.104.046722
Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy
Erratum in
- Biophys J. 2006 Jan 15;90(2):709
Abstract
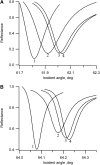
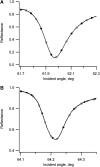
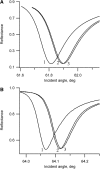
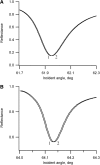
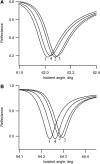
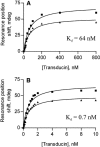
Flash photolysis studies have shown that the membrane lipid environment strongly influences the ability of rhodopsin to form the key metarhodopsin II intermediate. Here we have used plasmon-waveguide resonance (PWR) spectroscopy, an optical method sensitive to both mass and conformation, to probe the effects of lipid composition on conformational changes of rhodopsin induced by light and due to binding and activation of transducin (G(t)). Octylglucoside-solubilized rhodopsin was incorporated by detergent dilution into solid-supported bilayers composed either of egg phosphatidylcholine or various mixtures of a nonlamellar-forming lipid (dioleoylphosphatidylethanolamine; DOPE) together with a lamellar-forming lipid (dioleoylphosphatidylcholine; DOPC). Light-induced proteolipid conformational changes as a function of pH correlated well with previous flash photolysis studies, indicating that the PWR spectral shifts monitored metarhodopsin II formation. The magnitude of these effects, and hence the extent of the conformational transition, was found to be proportional to the DOPE content. Our data are consistent with previous suggestions that lipids having a negative spontaneous curvature favor elongation of rhodopsin during the activation process. In addition, measurements of the G(t)/rhodopsin interaction in a DOPC/DOPE (25:75) bilayer at pH 5 demonstrated that light activation increased the affinity for G(t) from 64 nM to 0.7 nM, whereas G(t) affinity for dark-adapted rhodopsin was unchanged. By contrast, in DOPC bilayers the affinity of G(t) for light-activated rhodopsin was only 18 nM at pH 5. Moreover exchange of GDP for GTP gamma S was also monitored by PWR spectroscopy. Only the light-activated receptor was able to induce this exchange which was unaffected by DOPE incorporation. These findings demonstrate that nonbilayer-forming lipids can alter functionally linked conformational changes of G-protein-coupled receptors in membranes, as well as their interactions with downstream effector proteins.
Figures





References
-
- Altenbach, C., K. Cai, H. G. Khorana, and W. L. Hubbell. 1999a. Structural features and light-dependent changes in the sequence 306–322 extending from helix VII to the palmitoylation sites in rhodopsin: a site-directed spin-labeling study. Biochemistry. 38:7931–7937. - PubMed
-
- Altenbach, C., K. Cai, J. Klein-Seetharaman, H. G. Khorana, and W. L. Hubbell. 2001a. Structure and function in rhodopsin: mapping light-dependent changes in distance between residue 65 in helix TM1 and residues in the sequence 306–319 at the cytoplasmic end of helix TM7 and in helix H8. Biochemistry. 40:15483–15492. - PubMed
-
- Altenbach, C., J. Klein-Seetharaman, K. Cai, H. G. Khorana, and W. L. Hubbell. 2001b. Structure and function in rhodopsin: mapping light-dependent changes in distance between residue 316 in helix 8 and residues in the sequence 60–75, covering the cytoplasmic end of helices TM1 and TM2 and their connection loop CL1. Biochemistry. 40:15493–15500. - PubMed
-
- Altenbach, C., J. Klein-Seetharaman, J. Hwa, H. G. Khorana, and W. L. Hubbell. 1999b. Structural features and light-dependent changes in the sequence 59–75 connecting helices I and II in rhodopsin: a site-directed spin-labeling study. Biochemistry. 38:7945–7949. - PubMed
-
- Alves, I. D., K. A. Ciano, V. Boguslavsky, E. Varga, Z. Salamon, H. I. Yamamura, V. J. Hruby, and G. Tollin. 2004. Selectivity, cooperativity and reciprocity in the interactions between the delta opioid receptor, its ligands and G-proteins. J. Biol. Chem. 279:44673–44682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

