Investigation of ligand binding to the multidrug resistance protein EmrE by isothermal titration calorimetry
- PMID: 15501941
- PMCID: PMC1305024
- DOI: 10.1529/biophysj.104.049247
Investigation of ligand binding to the multidrug resistance protein EmrE by isothermal titration calorimetry
Abstract
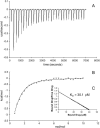
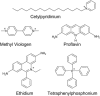
Escherichia coli multidrug resistance protein E (EmrE) is an integral membrane protein spanning the inner membrane of Escherichia coli that is responsible for this organism's resistance to a variety of lipophilic cations such as quaternary ammonium compounds (QACs) and interchelating dyes. EmrE is a 12-kDa protein of four transmembrane helices considered to be functional as a multimer. It is an efflux transporter that can bind and transport cytoplasmic QACs into the periplasm using the energy of the proton gradient across the inner membrane. Isothermal titration calorimetry provides information about the stoichiometry and thermodynamic properties of protein-ligand interactions, and can be used to monitor the binding of QACs to EmrE in different membrane mimetic environments. In this study the ligand binding to EmrE solubilized in dodecyl maltoside, sodium dodecyl sulfate and reconstituted into small unilamellar vesicles is examined by isothermal titration calorimetry. The binding stoichiometry of EmrE to drug was found to be 1:1, demonstrating that oligomerization of EmrE is not necessary for binding to drug. The binding of EmrE to drug was observed with the dissociation constant (K(D)) in the micromolar range for each of the drugs in any of the membrane mimetic environments. Thermodynamic properties demonstrated this interaction to be enthalpy-driven with similar enthalpies of 8-12 kcal/mol for each of the drugs in any of the membrane mimetics.
Figures
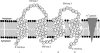


References
-
- Arkin, I. T., W. P. Russ, M. Lebendiker, and S. Schuldiner. 1996. Determining the secondary structure and orientation of EmrE, a multi-drug transporter, indicates a transmembrane four-helix bundle. Biochemistry. 35:7233–7238. - PubMed
-
- Bolhuis, H., H. W. van Veen, J. R. Brands, M. Putman, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1996. Energetics and mechanism of drug transport mediated by the lactococcal multidrug transporter LmrP. J. Biol. Chem. 271:24123–24128. - PubMed
-
- Butler, P., I. Ubarretxena-Belandia, T. Warne, and C. Tate. 2004. The Escherichia coli multidrug transporter EmrE is a dimer in the detergent-solubilised state. J. Mol. Biol. 340:797–808. - PubMed
-
- Dougherty, D. A. 1996. Cation-π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science. 271:163–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

