DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation
- PMID: 15502874
- PMCID: PMC521177
- DOI: 10.1371/journal.pbio.0020362
DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation
Abstract
Parkinson's disease (PD) pathology is characterized by the degeneration of midbrain dopamine neurons (DNs) ultimately leading to a progressive movement disorder in patients. The etiology of DN loss in sporadic PD is unknown, although it is hypothesized that aberrant protein aggregation and cellular oxidative stress may promote DN degeneration. Homozygous mutations in DJ-1 were recently described in two families with autosomal recessive inherited PD (Bonifati et al. 2003). In a companion article (Martinat et al. 2004), we show that mutations in DJ-1 alter the cellular response to oxidative stress and proteasomal inhibition. Here we show that DJ-1 functions as a redox-sensitive molecular chaperone that is activated in an oxidative cytoplasmic environment. We further demonstrate that DJ-1 chaperone activity in vivo extends to alpha-synuclein, a protein implicated in PD pathogenesis.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
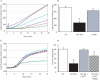
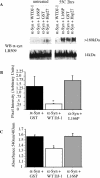

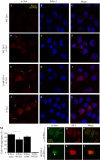
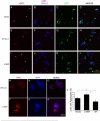
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. - PubMed
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Chelikani P, Donald LJ, Duckworth HW, Loewen PC. Hydroperoxidase II of Escherichia coli exhibits enhanced resistance to proteolytic cleavage compared to other catalases. Biochemistry. 2003;42:5729–5735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

