Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice
- PMID: 15504823
- PMCID: PMC2211860
- DOI: 10.1084/jem.20041328
Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice
Abstract
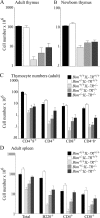
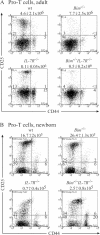
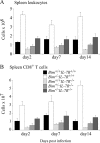
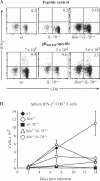
Interleukin (IL)-7 receptor (R) signaling is essential for T and B lymphopoiesis by promoting proliferation, differentiation, and survival of cells. Mice lacking either IL-7 or the IL-7Ralpha chain have abnormally low numbers of immature as well as mature T and B lymphocytes. Transgenic expression of the apoptosis inhibitor Bcl-2 rescues T cell development and function in IL-7Ralpha-deficient mice, indicating that activation of a proapoptotic Bcl-2 family member causes death of immature and mature T cells. BH3-only proteins such as Bim, which are distant proapoptotic members of the Bcl-2 family, are essential initiators of programmed cell death and stress-induced apoptosis. We generated Bim/IL-7Ralpha double deficient mice and found that loss of Bim significantly increased thymocyte numbers, restored near normal numbers of mature T cells in the blood and spleen, and enhanced cytotoxic T cell responses to virus infection in IL-7Ralpha-/- mice. These results indicate that Bim cooperates with other proapoptotic proteins in the death of IL-7-deprived T cell progenitors in vivo, but is the major inducer of this pathway to apoptosis in mature T cells. This indicates that pharmacological inhibition of Bim function might be useful for boosting immune responses in immunodeficient patients.
Figures

References
-
- Noguchi, M., Y. Nakamura, S.M. Russell, S.F. Ziegler, M. Tsang, X. Cao, and W.J. Leonard. 1993. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science. 262:1877–1880. - PubMed
-
- Morrissey, P.J., P. Conlon, K. Charrier, S. Braddy, A. Alpert, D. Williams, A.E. Namen, and D. Mochizuki. 1991. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J. Immunol. 147:561–568. - PubMed
-
- Fisher, A.G., C. Burdet, C. Bunce, M. Merkenschlager, and R. Ceredig. 1995. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int. Immunol. 7:415–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

