Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC
- PMID: 15504830
- PMCID: PMC525400
- DOI: 10.1128/AAC.48.11.4120-4129.2004
Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC
Abstract
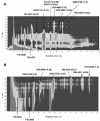
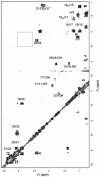
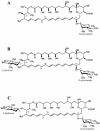
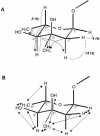
The gram-positive bacterium Streptomyces noursei ATCC 11455 produces a complex mixture of polyene macrolides generally termed nystatins. Although the structures for nystatins A(1) and A(3) have been reported, the identities of other components of the nystatin complex remain obscure. Analyses of the culture extract from the S. noursei wild type revealed the presence of several nystatin-related compounds for which chemical structures could be suggested on the basis of their molecular weights, their UV spectra, and knowledge of the nystatin biosynthetic pathway. Nuclear magnetic resonance (NMR) studies with one of these polyene macrolides identified it as a nystatin analogue containing a mycarose moiety at C-35. A similar investigation was performed with the culture extract of the ERD44 mutant, which has a genetically altered polyketide synthase (PKS) NysC and which was previously shown to produce a heptaene nystatin analogue. The latter compound, tentatively named S44HP, and its derivative, which contains two deoxysugar moieties, were purified; and their structures were confirmed by NMR analysis. Nystatin analogues with an expanded macrolactone ring were also observed in the extract of the ERD44 mutant, suggesting that the altered PKS can "stutter" during the polyketide chain assembly. These data provide new insights into the biosynthesis of polyene macrolide antibiotics and the functionalities of PKSs and post-PKS modification enzymes.
Figures


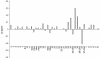
References
-
- Aparicio, J. F., R. Fouces, M. V. Mendes, N. Olivera, and J. F. Martín. 2000. A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem. Biol. 7:895-905. - PubMed
-
- Aparicio, J. F., P. Caffrey, J. A. Gil, and S. B. Zotchev. 2003. Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 61:179-188. - PubMed
-
- Bachmann, P., W. P. Aue, L. Müller, and R. R. Ernst. 1977. Phase separation in two-dimensional spectroscopy. J. Magn. Reson. 28:29-39.
-
- Bate, N., A. R. Butler, I. P. Smith, and E. Cundliffe. 2000. The mycarose-biosynthetic genes of Streptomyces fradiae, producer of tylosin. Microbiology 146:139-146. - PubMed
-
- Bax, A., and D. G. Davis. 1985. Practical aspects of two-dimensional transverse NOE spectroscopy. J. Magn. Reson. 63:207-213.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

