Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria
- PMID: 15504846
- PMCID: PMC525450
- DOI: 10.1128/AAC.48.11.4234-4239.2004
Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria
Erratum in
- Antimicrob Agents Chemother. 2005 Feb;49(2):871
Abstract
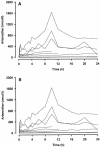
The first-dose pharmacokinetic properties of intramuscular (i.m.) artesunate (ARTS; 2.4 mg/kg immediately [stat], followed by 1.2 mg/kg i.m. daily) and artemether (ARM; 3.2 mg/kg i.m. stat, followed by 1.6 mg/kg i.m. daily) were compared in Vietnamese adults with severe falciparum malaria. A total of 19 patients were studied; 9 received ARTS, and 10 received ARM. ARTS was absorbed very rapidly; concentrations in plasma peaked between 1,362 and 8,388 nmol/liter (median, 5,710 nmol/liter) within 20 min of injection and then declined with a median (range) half-life (t(1/2)) of 30 (3 to 67) min. ARTS was hydrolyzed rapidly and completely to the biologically active metabolite dihydroartemisinin (DHA). Peak DHA concentrations in plasma ranged between 1,718 and 7,080 nmol/liter (median, 3,060 nmol/liter) and declined with a t(1/2) of 52 (26 to 69) min. In contrast, ARM was slowly and erratically absorbed. The absorption profile appeared biphasic. Maximum ARM concentrations in plasma ranged between 67 nmol/liter (a value close to the 50% inhibitory concentration for some Plasmodium falciparum isolates) and 1,631 nmol/liter (median, 574 nmol/liter) and occurred at a median (range) of 10 (1.5 to 24) h. There was relatively little conversion to DHA. After i.m. injection in cases of severe malaria, absorption of the water-soluble ARTS is rapid and extensive, whereas the oil-based ARM is slowly and erratically absorbed, with relatively little conversion to the more active DHA. On the basis of this pharmacological study, parenteral ARTS is preferable to ARM as an initial antimalarial therapy, particularly in the most seriously ill patients. These findings should be formally assessed by a randomized clinical trial.
Figures

References
-
- Artemether-Quinine Meta-Analysis Study Group. 2001. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:637-650. - PubMed
-
- Batty, K. T., T. M. E. Davis, L. T. Thu, T. Q. Binh, T. K. Anh, and K. F. Ilett. 1996. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J. Chromatogr. B 677:345-350. - PubMed
-
- Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive ARTS-mefloquine use. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. - PMC - PubMed
-
- Grace, J. M., A. J. Aguilar, K. M. Trotman, J. O. Peggins, and T. G. Brewer. 1998. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab. Disp. 26:313-317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

