Steady-state pharmacokinetics of a double-boosting regimen of saquinavir soft gel plus lopinavir plus minidose ritonavir in human immunodeficiency virus-infected adults
- PMID: 15504850
- PMCID: PMC525389
- DOI: 10.1128/AAC.48.11.4256-4262.2004
Steady-state pharmacokinetics of a double-boosting regimen of saquinavir soft gel plus lopinavir plus minidose ritonavir in human immunodeficiency virus-infected adults
Abstract
Management of treatment-experienced human immunodeficiency virus patients has become complex, and therapy may need to include two protease inhibitors at therapeutic doses. The objective of this study was to characterize the pharmacokinetics in serum of saquinavir (1,000 mg twice daily [b.i.d.]), lopinavir (400 mg b.i.d.), and ritonavir (100 mg b.i.d.) in a multidrug rescue therapy study and to investigate whether steady-state pharmacokinetics of lopinavir-ritonavir are affected by coadministration of saquinavir. Forty patients were included (25 given ritonavir, lopinavir, and saquinavir and 15 given ritonavir and lopinavir). The median pharmacokinetic parameters of lopinavir were as follows: area under the concentration-time curve from 0 to 12 h (AUC(0-12)), 85.1 microg/ml . h; maximum concentration of drug in serum (C(max)), 10.0 microg/ml; trough concentration of drug in serum (C(trough)), 7.3 microg/ml; and minimum concentration of drug in serum (C(min)), 5.5 microg/ml. Lopinavir concentrations were similar in patients with and without saquinavir. The median pharmacokinetic parameters for saquinavir were as follows: AUC(0-12), 22.9 microg/ml . h; C(max), 2.9 microg/ml; C(trough), 1.6 microg/ml; and C(min), 1.4 microg/ml. There was a strong linear correlation between lopinavir and ritonavir and between saquinavir and ritonavir concentrations in plasma. The correlation between lopinavir and saquinavir levels was weaker. We found higher saquinavir concentrations in women than in men, with no difference in lopinavir levels. Only patients with very high body weight presented lopinavir and saquinavir concentrations lower than the overall group. Ritonavir has a double-boosting function for both lopinavir and saquinavir, and in terms of pharmacokinetics, the drug doses selected seemed appropriate for combining these agents in a dual protease inhibitor-based antiretroviral regimen for patients with several prior virologic failures.
Figures
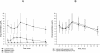
References
-
- Back, D., G. Gatti, C. Fletcher, R. Garaffo, R. Haubrich, R. Hoetelmans, M. Kurowski, A. Luber, C. Merry, and C. F. Perno. 2002. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS 16(Suppl. 1):S5-S37. - PubMed
-
- Baede-van Dijk, P. A., P. W. Hugen, C. P. Verweij-van Wissen, P. P. Koopmans, D. M. Burger, and Y. A. Hekster. 2001. Analysis of variation in plasma concentrations of nelfinavir and its active metabolite M8 in HIV-positive patients. AIDS 15:991-998. - PubMed
-
- Cameron, D., A. J. Japour, Y. Xu, A. Hsu, J. Mellors, C. Farthing, C. Cohen, D. Poretz, M. Markowitz, S. Follansbee, J. B. Angel, D. McMahon, D. Ho, V. Devanarayan, R. Rode, M. Salgo, D. J. Kempf, R. Granneman, J. M. Leonard, and E. Sun. 1999. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS 13:213-224. - PubMed
-
- Casado, J. L., M. J. Perez-Elias, A. Antela, R. Sabido, P. Marti-Belda, F. Dronda, J. Blazquez, and C. Quereda. 1998. Predictors of long-term response to protease inhibitor therapy in a cohort of HIV-infected patients. AIDS 12:F131-F135. - PubMed
-
- Casau, N. C., M. J. Glesby, S. Paul, and R. M. Gulick. 2003. Brief report: efficacy and treatment-limiting toxicity with the concurrent use of lopinavir/ritonavir and a third protease inhibitor in treatment-experienced HIV-infected patients. J. Acquir. Immune Defic. Syndr. 32:494-498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

