Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts
- PMID: 15504854
- PMCID: PMC525424
- DOI: 10.1128/AAC.48.11.4286-4292.2004
Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts
Abstract
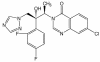
Albaconazole is an experimental triazole derivative with potent and broad-spectrum antifungal activity and a remarkably long half-life in dogs, monkeys, and humans. In the present work, we investigated the in vivo activity of this compound against two strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi, the causative agent of Chagas' disease, using dogs as hosts. The T. cruzi strains used in the study were previously characterized (murine model) as susceptible (strain Berenice-78) and partially resistant (strain Y) to the drugs currently in clinical use, nifurtimox and benznidazole. Our results demonstrated that albaconazole is very effective in suppressing the proliferation of the parasite and preventing the death of infected animals. Furthermore, the parasitological, PCR, serological, and proliferative assay results indicated parasitological cure indices of 25 and 100% among animals inoculated with T. cruzi strain Y when they were treated with albaconazole at 1.5 mg/kg of body weight/day for 60 and 90 days, respectively. On the other hand, although albaconazole given at 1.5 mg/kg/day was very effective in suppressing the proliferation of the parasite in animals infected with the Berenice-78 T. cruzi strain, no parasitological cure was observed among them, even when a longer treatment period (150 doses) was used. In conclusion, our results demonstrate that albaconazole has trypanocidal activity in vivo and is capable of inducing radical parasitological cure, although natural resistance to this compound was also indicated. Furthermore, the compound can be used in long-term treatment schemes (60 to 150 days) with minimal toxicity and thus represents a potentially useful candidate for the treatment of human Chagas' disease.
Figures



References
-
- Ávila, H. A., A. M. Gonçalves, N. C. Nehme, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Mol. Biochem. Parasitol. 42:175-188. - PubMed
-
- Ávila, H. A., D. S. Sigman, L. M. Cohen, R. C. Millikan, and L. Simpson. 1991. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolation from whole blood lysates: diagnosis of chronic Chagas' disease. Mol. Biochem. Parasitol. 40:211-222. - PubMed
-
- Bartrolí, J., E. Turmo, E. E. Algueró, M. L. Bomcompte, L. Vericat, J. Conte, J. Ramis, M. Merlos, J. Garcia-Rafanell, and J. Forn. 1998. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 41:1869-1882. - PubMed
-
- Brener, Z. 1984. Recent advances in the chemoterapy of Chagas disease. Mem. Inst. Oswaldo Cruz 79:149-155.
-
- Britto, C., M. A. Cardoso, P. Wincker, and C. M. A. Morel. 1993. Simple protocol for cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR) based diagnosis of chronic Chagas' disease. Mem. Inst. Oswaldo Cruz 88:171-172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

