Potential for interactions between caspofungin and nelfinavir or rifampin
- PMID: 15504857
- PMCID: PMC525392
- DOI: 10.1128/AAC.48.11.4306-4314.2004
Potential for interactions between caspofungin and nelfinavir or rifampin
Abstract
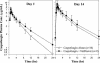
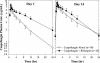
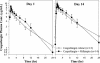
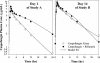
The potential for interactions between caspofungin and nelfinavir or rifampin was evaluated in two parallel-panel studies. In study A, healthy subjects received a 14-day course of caspofungin alone (50 mg administered intravenously [IV] once daily) (n = 10) or with nelfinavir (1,250 mg administered orally twice daily) (n = 9) or rifampin (600 mg administered orally once daily) (n = 10). In study B, 14 subjects received a 28-day course of rifampin (600 mg administered orally once daily), with caspofungin (50 mg administered IV once daily) coadministered on the last 14 days, and 12 subjects received a 14-day course of caspofungin alone (50 mg administered IV once daily). The coadministration/administration alone geometric mean ratio for the caspofungin area under the time-concentration profile calculated for the 24-h period following dosing [AUC(0-24)] was as follows (values in parentheses are 90% confidence intervals [CIs]): 1.08 (0.93-1.26) for nelfinavir, 1.12 (0.97-1.30) for rifampin (study A), and 1.01 (0.91-1.11) for rifampin (study B). The shape of the caspofungin plasma profile was altered by rifampin, resulting in a 14 to 31% reduction in the trough concentration at 24 h after dosing (C(24h)), consistent with a net induction effect at steady state. Both the AUC and the C(24h) were elevated in the initial days of rifampin coadministration in study A (61 and 170% elevations, respectively, on day 1) but not in study B, consistent with transient net inhibition prior to full induction. The coadministration/administration alone geometric mean ratio for the rifampin AUC(0-24) on day 14 was 1.07 (90% CI, 0.83-1.38). Nelfinavir does not meaningfully alter caspofungin pharmacokinetics. Rifampin both inhibits and induces caspofungin disposition, resulting in a reduced C(24h) at steady state. An increase in the caspofungin dose to 70 mg, administered daily, should be considered when the drug is coadministered with rifampin.
Figures


Similar articles
-
Lack of pharmacokinetic drug interaction between oral posaconazole and caspofungin or micafungin.J Clin Pharmacol. 2011 Jan;51(1):84-92. doi: 10.1177/0091270009360982. Epub 2010 May 20. J Clin Pharmacol. 2011. PMID: 20489029 Clinical Trial.
-
Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations.J Clin Pharmacol. 2007 Aug;47(8):951-61. doi: 10.1177/0091270007303764. J Clin Pharmacol. 2007. PMID: 17660480 Clinical Trial.
-
Assessing the antifungal activity, pharmacokinetics, and tissue distribution of amphotericin B following the administration of Abelcet and AmBisome in combination with caspofungin to rats infected with Aspergillus fumigatus.J Pharm Sci. 2007 Jul;96(7):1737-47. doi: 10.1002/jps.20801. J Pharm Sci. 2007. PMID: 17080414
-
Pharmacokinetics and pharmacodynamics of saquinavir in pediatric patients with human immunodeficiency virus infection.Clin Pharmacol Ther. 2002 Mar;71(3):122-30. doi: 10.1067/mcp.2002.121423. Clin Pharmacol Ther. 2002. PMID: 11907486 Review.
-
The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications.Pharmacology. 2006;78(4):161-77. doi: 10.1159/000096348. Epub 2006 Oct 17. Pharmacology. 2006. PMID: 17047411 Review.
Cited by
-
Invasive fungal infections in the ICU: how to approach, how to treat.Molecules. 2014 Jan 17;19(1):1085-119. doi: 10.3390/molecules19011085. Molecules. 2014. PMID: 24445340 Free PMC article. Review.
-
Pharmacokinetics/Pharmacodynamics of Caspofungin in Plasma and Peritoneal Fluid of Liver Transplant Recipients.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0118721. doi: 10.1128/AAC.01187-21. Epub 2021 Oct 18. Antimicrob Agents Chemother. 2022. PMID: 34662185 Free PMC article.
-
Clinically important pharmacokinetic drug-drug interactions with antibacterial agents.Rev Esp Quimioter. 2024 Aug;37(4):299-322. doi: 10.37201/req/037.2024. Epub 2024 Jun 5. Rev Esp Quimioter. 2024. PMID: 38840420 Free PMC article. Review.
-
In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin.Antimicrob Agents Chemother. 2009 Mar;53(3):1149-56. doi: 10.1128/AAC.01279-08. Epub 2008 Nov 24. Antimicrob Agents Chemother. 2009. PMID: 19029327 Free PMC article.
-
Individualized Pharmaceutical Care for Antifungal Therapy in a Patient with Aspergillus tubingensis Spondylitis After Discontinuation of Rifampicin: A Case Report.Infect Drug Resist. 2023 Jul 4;16:4349-4356. doi: 10.2147/IDR.S417604. eCollection 2023. Infect Drug Resist. 2023. PMID: 37424673 Free PMC article.
References
-
- Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. - PMC - PubMed
-
- Bouffard, F. A., R. A. Zambias, J. F. Dropinski, J. M. Balkovec, M. L. Hammond, G. K. Abruzzo, K. F. Bartizal, J. A. Marrinan, and M. B. Kurtz. 1994. Synthesis and antifungal activity of novel cationic pneumocandin Bo derivatives. J. Med. Chem. 37:222-225. - PubMed
-
- Fattinger, K., V. Cattori, B. Hagenbuch, P. J. Meier, B. Stieger. 2000. Rifamycin SV and rifampicin exhibit differential inhibition of the hepatic rat organic anion transporting polypeptides, Oatp1 and Oatp2. Hepatology 32:82-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

