Dose-dependent resorption of quinine after intrarectal administration to children with moderate Plasmodium falciparum malaria
- PMID: 15504872
- PMCID: PMC525409
- DOI: 10.1128/AAC.48.11.4422-4426.2004
Dose-dependent resorption of quinine after intrarectal administration to children with moderate Plasmodium falciparum malaria
Abstract
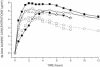
The pharmacokinetics of increasing doses of an intrarectal Cinchona alkaloid combination containing 96.1% quinine, 2.5% quinidine, 0.68% cinchonine, and 0.67% cinchonidine (Quinimax) was compared to that of parenteral regimens in 60 children with moderate malaria. Quinine exhibited a nonlinear pharmacokinetics, suggesting a saturation of rectal resorption. When early rejections appeared, blood quinine concentrations decreased by 30 to 50% and were restored by an immediate half-dose administration of the drug. Rectal administration of doses of 16 or 20 mg/kg of body weight led to concentration-time profiles in blood similar to those of parenteral regimens and could be an early treatment of childhood malaria.
Figures

Similar articles
-
Intrarectal Quinimax (an association of Cinchona alkaloids) for the treatment of Plasmodium falciparum malaria in children in Niger: efficacy and pharmacokinetics.Trans R Soc Trop Med Hyg. 1995 Jul-Aug;89(4):418-21. doi: 10.1016/0035-9203(95)90036-5. Trans R Soc Trop Med Hyg. 1995. PMID: 7570885
-
Pharmacokinetics of quinine, quinidine and Cinchonine when given as combination.Southeast Asian J Trop Med Public Health. 1992 Dec;23(4):773-6. Southeast Asian J Trop Med Public Health. 1992. PMID: 1298088 Clinical Trial.
-
Efficacy and pharmacokinetics of a new intrarectal quinine formulation in children with Plasmodium falciparum malaria.Br J Clin Pharmacol. 1996 May;41(5):389-95. doi: 10.1046/j.1365-2125.1996.03246.x. Br J Clin Pharmacol. 1996. PMID: 8735679 Free PMC article. Clinical Trial.
-
Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications.Clin Pharmacokinet. 1996 Apr;30(4):263-99. doi: 10.2165/00003088-199630040-00002. Clin Pharmacokinet. 1996. PMID: 8983859 Review.
-
Artemether for severe malaria.Cochrane Database Syst Rev. 2019 Jun 18;6(6):CD010678. doi: 10.1002/14651858.CD010678.pub3. Cochrane Database Syst Rev. 2019. PMID: 31210357 Free PMC article.
Cited by
-
Intrarectal quinine versus intravenous or intramuscular quinine for treating Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2009 Jan 21;2009(1):CD004009. doi: 10.1002/14651858.CD004009.pub3. Cochrane Database Syst Rev. 2009. PMID: 19160229 Free PMC article.
-
Formulation of quinine suppository for initiation of early treatment of malaria - a preliminary study.Malariaworld J. 2012 Dec 10;3:14. doi: 10.5281/zenodo.10997879. eCollection 2012. Malariaworld J. 2012. PMID: 38854879 Free PMC article.
-
Clinical pharmacokinetics of quinine and its relationship with treatment outcomes in children, pregnant women, and elderly patients, with uncomplicated and complicated malaria: a systematic review.Malar J. 2022 Feb 10;21(1):41. doi: 10.1186/s12936-022-04065-1. Malar J. 2022. PMID: 35144612 Free PMC article.
-
Safety and efficacy of rectal compared with intramuscular quinine for the early treatment of moderately severe malaria in children: randomised clinical trial.BMJ. 2006 May 6;332(7549):1055-9. doi: 10.1136/bmj.332.7549.1055. BMJ. 2006. PMID: 16675812 Free PMC article. Clinical Trial.
-
Pilot feasibility study of an emergency paediatric kit for intra-rectal quinine administration used by the personnel of community-based health care units in Senegal.Malar J. 2007 Nov 15;6:152. doi: 10.1186/1475-2875-6-152. Malar J. 2007. PMID: 18005442 Free PMC article. Clinical Trial.
References
-
- Assimadi, J. K., A. D. Gbadoe, O. Agbodjan-Djossou, S. E. Larsen, K. Kusiaku, K. Lawson-Evi, D. Redah, A. Adjogble, and A. Gayibor. 2002. Diluted injectable quinine in the intramuscular and intrarectal route: comparative efficacy and tolerance in malaria treatment for children. Med. Trop. 62:158-162. (In French.) - PubMed
-
- Barennes, H., F. Kahitani, E. Pussard, F. Clavier, D. Meynard, S. Njifountawouo, and F. Verdier. 1995. Intrarectal Quinimax (an association of Cinchona alkaloids) for the treatment of Plasmodium falciparum malaria in children in Niger: efficacy and pharmacokinetics. Trans. R. Soc. Trop. Med. Hyg. 89:418-421. - PubMed
-
- Barennes, H., J. Munjakazi, F. Verdier, F. Clavier, and E. Pussard. 1998. An open randomized clinical study of intrarectal versus infused Quinimax for the treatment of childhood cerebral malaria in Niger. Trans. R. Soc. Trop. Med. Hyg. 92:437-440. - PubMed
-
- Barennes, H., H. Sterlingot, N. Nagot, H. Meda, M. Kaboré, M. Sanou, B. Nacro, P. Bourée, and E. Pussard. 2003. Intrarectal pharmacokinetics of two formulations of quinine in children with falciparum malaria. Eur. J. Clin. Pharmacol. 58:649-652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

