Developmental regulation of calcium-dependent feedback in Xenopus rods
- PMID: 15504902
- PMCID: PMC2234010
- DOI: 10.1085/jgp.200409162
Developmental regulation of calcium-dependent feedback in Xenopus rods
Abstract
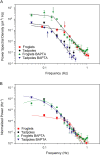
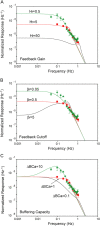
The kinetics of activation and inactivation in the phototransduction pathway of developing Xenopus rods were studied. The gain of the activation steps in transduction (amplification) increased and photoresponses became more rapid as the rods matured from the larval to the adult stage. The time to peak was significantly shorter in adults (1.3 s) than tadpoles (2 s). Moreover, adult rods recovered twice as fast from saturating flashes than did larval rods without changes of the dominant time constant (2.5 s). Guanylate cyclase (GC) activity, determined using IBMX steps, increased in adult rods from approximately 1.1 s(-1) to 3.7 s(-1) 5 s after a saturating flash delivering 6,000 photoisomerizations. In larval rods, it increased from 1.8 s(-1) to 4.0 s(-1) 9 s after an equivalent flash. However, the ratio of amplification to the measured dark phosphodiesterase activity was constant. Guanylate cyclase-activating protein (GCAP1) levels and normalized Na+/Ca2+, K+ exchanger currents were increased in adults compared with tadpoles. Together, these results are consistent with the acceleration of the recovery phase in adult rods via developmental regulation of calcium homeostasis. Despite these large changes, the single photon response amplitude was approximately 0.6 pA throughout development. Reduction of calcium feedback with BAPTA increased adult single photon response amplitudes threefold and reduced its cutoff frequency to that observed with tadpole rods. Linear mathematical modeling suggests that calcium-dependent feedback can account for the observed differences in the power spectra of larval and adult rods. We conclude that larval Xenopus maximize sensitivity at the expense of slower response kinetics while adults maximize response kinetics at the expense of sensitivity.
Figures



Similar articles
-
Activation and quenching of the phototransduction cascade in retinal cones as inferred from electrophysiology and mathematical modeling.Mol Vis. 2015 Mar 7;21:244-63. eCollection 2015. Mol Vis. 2015. PMID: 25866462 Free PMC article.
-
Toward a unified model of vertebrate rod phototransduction.Vis Neurosci. 2005 Jul-Aug;22(4):417-36. doi: 10.1017/S0952523805224045. Vis Neurosci. 2005. PMID: 16212700 Free PMC article.
-
Light responses and light adaptation in rat retinal rods at different temperatures.J Physiol. 2005 Sep 15;567(Pt 3):923-38. doi: 10.1113/jphysiol.2005.090662. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037091 Free PMC article.
-
Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: facts and models.Prog Retin Eye Res. 2012 Sep;31(5):442-66. doi: 10.1016/j.preteyeres.2012.05.002. Epub 2012 May 29. Prog Retin Eye Res. 2012. PMID: 22658984 Free PMC article. Review.
-
The flash response of rods in vivo.Prog Brain Res. 2001;131:369-81. doi: 10.1016/s0079-6123(01)31030-0. Prog Brain Res. 2001. PMID: 11420956 Review. No abstract available.
Cited by
-
The phototransduction machinery in the rod outer segment has a strong efficacy gradient.Proc Natl Acad Sci U S A. 2015 May 19;112(20):E2715-24. doi: 10.1073/pnas.1423162112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941368 Free PMC article.
-
Shedding light on cones.J Gen Physiol. 2006 Apr;127(4):355-8. doi: 10.1085/jgp.200609528. J Gen Physiol. 2006. PMID: 16567463 Free PMC article. Review. No abstract available.
-
Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors.J Comp Neurol. 2010 Feb 15;518(4):526-46. doi: 10.1002/cne.22236. J Comp Neurol. 2010. PMID: 20029995 Free PMC article.
-
Inverted photocurrent responses from amphibian rod photoreceptors: role of membrane voltage in response recovery.J Physiol. 2005 Jul 15;566(Pt 2):455-66. doi: 10.1113/jphysiol.2005.090258. Epub 2005 May 26. J Physiol. 2005. PMID: 15919708 Free PMC article.
-
Reprogramming progeny cells of embryonic RPE to produce photoreceptors: development of advanced photoreceptor traits under the induction of neuroD.Invest Ophthalmol Vis Sci. 2008 Sep;49(9):4145-53. doi: 10.1167/iovs.07-1380. Epub 2008 May 9. Invest Ophthalmol Vis Sci. 2008. PMID: 18469196 Free PMC article.
References
-
- Aho, A.C., K. Donner, C. Hyden, L.O. Larsen, and T. Reuter. 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 334:348–350. - PubMed
-
- Arshavsky, V.Y., T.D. Lamb, and E.N. Pugh Jr. 2002. G proteins and phototransduction. Annu. Rev. Physiol. 64:153–187. - PubMed
-
- Bowes, C., T. van Veen, and D.B. Farber. 1988. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp. Eye Res. 47:369–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

