A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them
- PMID: 15504916
- PMCID: PMC2172560
- DOI: 10.1083/jcb.200404129
A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them
Abstract
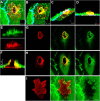
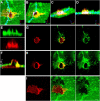
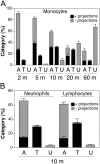
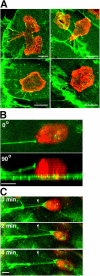
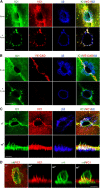
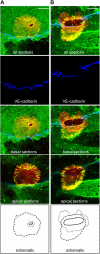
The basic route and mechanisms for leukocyte migration across the endothelium remain poorly defined. We provide definitive evidence for transcellular (i.e., through individual endothelial cells) diapedesis in vitro and demonstrate that virtually all, both para- and transcellular, diapedesis occurs in the context of a novel "cuplike" transmigratory structure. This endothelial structure was comprised of highly intercellular adhesion molecule-1- and vascular cell adhesion molecule-1-enriched vertical microvilli-like projections that surrounded transmigrating leukocytes and drove redistribution of their integrins into linear tracks oriented parallel to the direction of diapedesis. Disruption of projections was highly correlated with inhibition of transmigration. These findings suggest a novel mechanism, the "transmigratory cup", by which the endothelium provides directional guidance to leukocytes for extravasation.
Figures


References
-
- Adamson, P., S. Etienne, P.O. Couraud, V. Calder, and J. Greenwood. 1999. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J. Immunol. 162:2964–2973. - PubMed
-
- Aurrand-Lions, M., C. Johnson-Leger, and B.A. Imhof. 2002. The last molecular fortress in leukocyte trans-endothelial migration. Nat. Immunol. 3:116–118. - PubMed
-
- Barreiro, O., M. Yanez-Mo, J.M. Serrador, M.C. Montoya, M. Vicente-Manzanares, R. Tejedor, H. Furthmayr, and F. Sanchez-Madrid. 2002. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 157:1233–1245. - PMC - PubMed
-
- Bianchi, E., J.R. Bender, F. Blasi, and R. Pardi. 1997. Through and beyond the wall: late steps in leukocyte transendothelial migration. Immunol. Today. 18:586–591. - PubMed

