AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner
- PMID: 15505206
- PMCID: PMC524823
- DOI: 10.1073/pnas.0401502101
AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner
Abstract
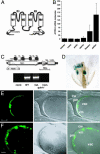
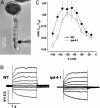
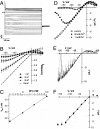
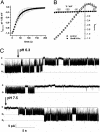
The Arabidopsis tandem-pore K(+) (TPK) channels displaying four transmembrane domains and two pore regions share structural homologies with their animal counterparts of the KCNK family. In contrast to the Shaker-like Arabidopsis channels (six transmembrane domains/one pore region), the functional properties and the biological role of plant TPK channels have not been elucidated yet. Here, we show that AtTPK4 (KCO4) localizes to the plasma membrane and is predominantly expressed in pollen. AtTPK4 (KCO4) resembles the electrical properties of a voltage-independent K(+) channel after expression in Xenopus oocytes and yeast. Hyperpolarizing as well as depolarizing membrane voltages elicited instantaneous K(+) currents, which were blocked by extracellular calcium and cytoplasmic protons. Functional complementation assays using a K(+) transport-deficient yeast confirmed the biophysical and pharmacological properties of the AtTPK4 channel. The features of AtTPK4 point toward a role in potassium homeostasis and membrane voltage control of the growing pollen tube. Thus, AtTPK4 represents a member of plant tandem-pore-K(+) channels, resembling the characteristics of its animal counterparts as well as plant-specific features with respect to modulation of channel activity by acidosis and calcium.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

