Antigen-independent memory CD8 T cells do not develop during chronic viral infection
- PMID: 15505208
- PMCID: PMC524220
- DOI: 10.1073/pnas.0407192101
Antigen-independent memory CD8 T cells do not develop during chronic viral infection
Abstract
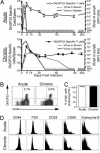
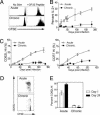
Memory T cells can persist for extended periods in the absence of antigen, and long-term T cell immunity is often seen after acute infections. Paradoxically, there have been observations suggesting that T cell memory may be antigen-dependent during chronic infections. To elucidate the underlying mechanisms we have compared memory CD8 T cell differentiation during an acute versus chronic infection by using the mouse model of infection with lymphocytic choriomeningitis virus. We found that during a chronic infection virus-specific CD8 T cells failed to acquire the cardinal memory T cell property of long-term antigen-independent persistence. These chronically stimulated CD8 T cells were unable to undergo homeostatic proliferation, responded poorly to IL-7 and IL-15, and expressed reduced levels of the IL-7 and IL-15 receptors, thus providing a possible mechanism for the inability of these cells to persist long term in the absence of antigen. In striking contrast, virus-specific memory CD8 T cells that developed after an acute lymphocytic choriomeningitis virus infection could persist without antigen, were capable of self-renewal because of homeostatic proliferation, responded efficiently to IL-7 and IL-15, and expressed high levels of receptors for these two cytokines. Thus, memory CD8 T cells generated after acute infections are likely to have a competitive advantage over CD8 T cells that develop during chronic infections. These findings raise concerns about using vaccines that may persist and also suggest that there may be limitations and challenges in designing effective immunological interventions for the treatment of chronic infections and tumors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

