EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion
- PMID: 15505211
- PMCID: PMC528746
- DOI: 10.1073/pnas.0403668101
EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion
Abstract
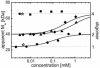
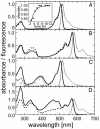
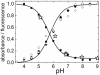
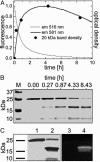
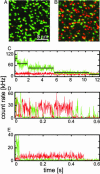
A gene encoding a fluorescent protein from the stony coral Lobophyllia hemprichii has been cloned in Escherichia coli and characterized by biochemical and biophysical methods. The protein, which we named EosFP, emits strong green fluorescence (516 nm) that changes to red (581 nm) upon near-UV irradiation at approximately 390 nm because of a photo-induced modification involving a break in the peptide backbone next to the chromophore. Single-molecule fluorescence spectroscopy shows that the wild type of EosFP is tetrameric, with strong Forster resonance coupling among the individual fluorophores. We succeeded in breaking up the tetramer into AB and AC subunit dimers by introducing the single point mutations V123T and T158H, respectively, and the combination of both mutations yielded functional monomers. Fusion constructs with a variety of proteins were prepared and expressed in human cells, showing that normal biological functions were retained. The possibility to locally change the emission wavelength by focused UV light makes EosFP a superb marker for experiments aimed at tracking the movements of biomolecules within the living cell.
Figures

References
-
- Prasher, D. C., Eckenrode, V. K., Ward, W. W., Prendergast, F. G. & Cormier, M. J. (1992) Gene 111, 229–233. - PubMed
-
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C. (1994) Science 263, 802–805. - PubMed
-
- Tsien, R. Y. (1998) Annu. Rev. Biochem. 67, 509–544. - PubMed
-
- Wiedenmann, J., Röcker, C. & Funke, W. (1999) in Verhandlungen der Gesellschaft für Ökologie, ed. Pfadenhauer, J. (Springer, Heidelberg), Vol. 29, pp. 497–503.
-
- Wiedenmann, J. (1997) in Offenlegungsschrift DE 197 18 640 A1 (German Patent and Trade Mark Office, Munich), pp. 1–18.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

