Facile isolation and the characterization of human retinal stem cells
- PMID: 15505221
- PMCID: PMC524825
- DOI: 10.1073/pnas.0401596101
Facile isolation and the characterization of human retinal stem cells
Abstract
This study identifies and characterizes retinal stem cells (RSCs) in early postnatal to seventh-decade human eyes. Different subregions of human eyes were dissociated and cultured by using a clonal sphere-forming assay. The stem cells were derived only from the pars plicata and pars plana of the retinal ciliary margin, at a frequency of approximately 1:500. To test for long-term self-renewal, both the sphere assay and monolayer passaging were used. By using the single sphere passaging assay, primary spheres were dissociated and replated, and individual spheres demonstrated 100% self-renewal, with single spheres giving rise to one or more new spheres in each subsequent passage. The clonal retinal spheres were plated under differentiation conditions to assay the differentiation potential of their progeny. The spheres were produced all of the different retinal cell types, demonstrating multipotentiality. Therefore, the human eye contains a small population of cells (approximately equal to 10,000 cells per eye) that have retinal stem-cell characteristics (proliferation, self-renewal, and multipotentiality). To test the in vivo potential of the stem cells and their progeny, we transplanted dissociated human retinal sphere cells, containing both stem cells and progenitors, into the eyes of postnatal day 1 NOD/SCID mice and embryonic chick eyes. The progeny of the RSCs were able to survive, migrate, integrate, and differentiate into the neural retina, especially as photoreceptors. Their facile isolation, integration, and differentiation suggest that human RSCs eventually may be valuable in treating human retinal diseases.
Figures
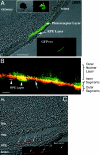
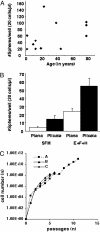

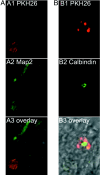
References
-
- Tropepe, V., Coles, B. L. K., Chiasson, B. J., Horsford, D. J., Elia, A. J., McInnes, R. R., van der Kooy, D. (2000) Science 287, 2032-2036. - PubMed
-
- Ahmad, I., Tang, L. & Pham, H. (2000) Biochem. Biophy. Res. Commun. 270, 517-521. - PubMed
-
- Yang, P., Seiler, M. J., Aramant, R. B. & Whittemore, S. R. (2002) Exp. Neurol. 177, 326-331. - PubMed
-
- Jensen, A. M. & Raff, M. C. (1997) Dev. Biol. 188, 267-279. - PubMed
-
- Vescovi, A. L., Gritti, A., Galli, R. & Parati, E. A. (1999) J. Neurotrauma 16, 689-693. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

