Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation
- PMID: 15505339
- PMCID: PMC3957812
- DOI: 10.1369/jhc.3A6241.2004
Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation
Abstract
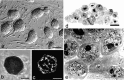
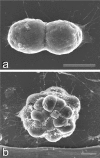
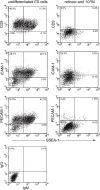
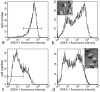
We examined the distribution of cell adhesion-related molecules (CAMs) among mouse embryonic stem (ES) cells and the spatial distribution on cell surfaces before and during differentiation. The cell-cell heterogeneity of SSEA-1, PECAM-1, and ICAM-1 among the undifferentiated cells in the ES cell colonies was evident by immunohistochemistry and immuno-SEM, supporting the flow cytometry findings. In contrast, most undifferentiated ES cells strongly expressed CD9. SSEA-1 was located preferentially on the edge of low protuberances and microvilli and formed clusters or linear arrays of 3-20 particles. PECAM-1 and ICAM-1 were randomly localized on the free cell surfaces, whereas CD9 was preferentially localized on the microvilli or protuberances, especially in the cell periphery. Both the SSEA-1(+) fraction and the SSEA-1(-) fraction of magnetic cell sorting (MACS) formed undifferentiated colonies after plating. Flow cytometry showed that these populations reverted separately again to a culture with a mixed phenotype. Differentiation induced by retinoic acid downregulated the expression of all CAMs. Immuno-SEM showed decreases of SSEA-1 in the differentiated ES cells, although some clustering still remained. Our findings help to elucidate the significance of these molecules in ES cell maintenance and differentiation and suggest that cell surface antigens may be useful for defining the phenotype of undifferentiated and differentiated ES cells.
Figures

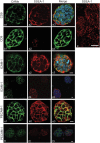

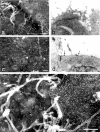
References
-
- Belaaloui G, Imbert AM, Bardin F, Tonnelle C, Dubreuil P, Lopez M, Chabannon C. (2003) Functional characterization of human CD34+ cells that express low or high levels of the membrane antigen CD111 (nectin 1). Leukemia 17:1137–1145 - PubMed
-
- Bird JM, Kimber SJ. (1984) Oligosaccharides containing fucose linked alpha (1–3) and alpha(1–4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol 104:449–460 - PubMed
-
- Connolly DT, Townsend RR, Kawaguchi K, Bell WR, Lee YC. (1982) Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem 257:939–945 - PubMed
-
- Cook GA, Longhurst CM, Grgurevich S, Cholera S, Crossno JT, Jr, Jennings LK. (2002) Identification of CD9 extracellular domains important in regulation of CHO cell adhesion to fibronectin and fibronectin pericellular matrix assembly. Blood 100:4502–4511 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

