Phase II study of S-1 in patients with advanced biliary tract cancer
- PMID: 15505626
- PMCID: PMC2410053
- DOI: 10.1038/sj.bjc.6602208
Phase II study of S-1 in patients with advanced biliary tract cancer
Abstract
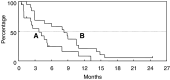
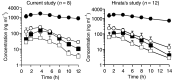
The aim of this study was to investigate the efficacy and safety of an oral fluoropyrimidine derivative, S-1, in patients with advanced biliary tract cancer. Patients with pathologically confirmed advanced biliary tract cancer, a measurable lesion, and no history of radiotherapy or chemotherapy were enrolled. S-1 was administered orally (40 mg m(-2) b.i.d.) for 28 days, followed by a 14-day rest period. A pharmacokinetic study was performed on day 1 in the initial eight patients. In all, 19 consecutive eligible patients were enrolled in the study between July 2000 and January 2002. The site of the primary tumour was the gallbladder (n=16), the extrahepatic bile ducts (n=2), and the ampulla of Vater (n=1). A median of two courses of treatment (range, 1-12) was administered. Four patients achieved a partial response, giving an overall response rate of 21.1%. The median time-to-progression and median overall survival period were 3.7 and 8.3 months, respectively. Although grade 3 anorexia and fatigue occurred in two patients each (10.5%), no grade 4 toxicities were observed. The pharmacokinetic parameters after a single oral administration of S-1 were similar to those of patients with other cancers. S-1 exhibits definite antitumour activity and is well tolerated in patients with advanced biliary tract cancer.
Figures
References
-
- Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39: 1264–1270 - PubMed
-
- Cohen SJ, Leichman CG, Yeslow G, Beard M, Proefrock A, Roedig B, Damle B, Letrent SP, DeCillis AP, Meropol NJ (2002) Phase I and pharmacokinetic study of once daily oral administration of S-1 in patients with advanced cancer. Clin Cancer Res 8: 2116–2122 - PubMed
-
- Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, Fandi L, Zarba J, Armand JP (1998) Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol 9: 653–656 - PubMed
-
- Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C, Ahumada M (2001) A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 12: 1403–1406 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical