Uptake of denatured collagen into hepatic stellate cells: evidence for the involvement of urokinase plasminogen activator receptor-associated protein/Endo180
- PMID: 15506989
- PMCID: PMC1134930
- DOI: 10.1042/BJ20040966
Uptake of denatured collagen into hepatic stellate cells: evidence for the involvement of urokinase plasminogen activator receptor-associated protein/Endo180
Abstract
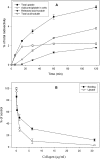
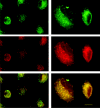
Tissue remodelling is dependent on the integration of signals that control turnover of ECM (extracellular matrix). Breakdown and endocytosis of collagen, a major component of the ECM, is central to this process. Whereas controlled secretion of matrix-degrading enzymes (such as matrix metalloproteinases) has long been known to mediate ECM breakdown, it is becoming clear that uPARAP/Endo180 (where uPARAP stands for urokinase plasminogen activator receptor-associated protein) serves as a receptor that mediates endocytosis of collagen by several types of cells. In the liver, the stellate cells play a major role in turnover of ECM including collagens. These cells synthesize various collagens and also produce matrix metalloproteinases. In the present study, we investigated the capacity of rat hepatic stellate cells to endocytose and degrade 125I-labelled heat-denatured collagen I. It was found that the collagen is efficiently taken up and degraded by these cells. Degradation was inhibited by inhibitors of lysosomal proteases (leupeptin and E-64d) and the vacuolar proton pump (concanamycin A), indicating that it takes place in lysosomes. Furthermore, endocytosed FITC-labelled collagen was shown to reach late endocytic compartments in which it colocalized with LysoTracker (a marker of late endocytic compartments). Competition experiments showed that uPA and unlabelled collagen are capable of inhibiting binding and uptake of [125I]collagen in a dose-dependent manner. Moreover, Western-blot analysis of cell lysate (using a polyclonal rabbit human-Endo180 antiserum) revealed a single band at 180 kDa. In addition, the antiserum was capable of reducing [125I]collagen binding to the cell surface. Finally, using two primers designed from the human uPARAP/Endo180 mRNA sequence, the expression of uPARAP/Endo180 mRNA was detected by reverse transcriptase-PCR. These results together suggest that uPARAP/Endo180 mediates endocytosis of collagen in rat liver stellate cells.
Figures





Similar articles
-
Up-regulation of uPARAP/Endo180 during culture activation of rat hepatic stellate cells and its presence in hepatic stellate cell lines from different species.BMC Cell Biol. 2009 May 11;10:39. doi: 10.1186/1471-2121-10-39. BMC Cell Biol. 2009. PMID: 19432973 Free PMC article.
-
The collagen receptor uPARAP/Endo180.Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2103-14. doi: 10.2741/3365. Front Biosci (Landmark Ed). 2009. PMID: 19273187 Review.
-
uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion.J Cell Biol. 2003 Mar 31;160(7):1009-15. doi: 10.1083/jcb.200211091. J Cell Biol. 2003. PMID: 12668656 Free PMC article.
-
Endocytic collagen degradation: a novel mechanism involved in protection against liver fibrosis.J Pathol. 2012 May;227(1):94-105. doi: 10.1002/path.3981. Epub 2012 Feb 17. J Pathol. 2012. PMID: 22294280
-
uPARAP/Endo180: a multifaceted protein of mesenchymal cells.Cell Mol Life Sci. 2022 Apr 22;79(5):255. doi: 10.1007/s00018-022-04249-7. Cell Mol Life Sci. 2022. PMID: 35460056 Free PMC article. Review.
Cited by
-
You Say You Want a Resolution (of Fibrosis).Am J Respir Cell Mol Biol. 2020 Oct;63(4):424-435. doi: 10.1165/rcmb.2020-0182TR. Am J Respir Cell Mol Biol. 2020. PMID: 32640171 Free PMC article. Review.
-
Cellular uptake of collagens and implications for immune cell regulation in disease.Cell Mol Life Sci. 2020 Aug;77(16):3161-3176. doi: 10.1007/s00018-020-03481-3. Epub 2020 Feb 25. Cell Mol Life Sci. 2020. PMID: 32100084 Free PMC article. Review.
-
uPARAP expression during murine lung development.Gene Expr Patterns. 2008 Sep;8(7-8):486-93. doi: 10.1016/j.gep.2008.06.006. Epub 2008 Jun 28. Gene Expr Patterns. 2008. PMID: 18639653 Free PMC article.
-
Up-regulation of uPARAP/Endo180 during culture activation of rat hepatic stellate cells and its presence in hepatic stellate cell lines from different species.BMC Cell Biol. 2009 May 11;10:39. doi: 10.1186/1471-2121-10-39. BMC Cell Biol. 2009. PMID: 19432973 Free PMC article.
-
Endocytosis of collagen by hepatic stellate cells regulates extracellular matrix dynamics.Am J Physiol Cell Physiol. 2014 Oct 1;307(7):C622-33. doi: 10.1152/ajpcell.00086.2014. Epub 2014 Jul 30. Am J Physiol Cell Physiol. 2014. PMID: 25080486 Free PMC article.
References
-
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int. Rev. Cytol. 1980;66:303–353. - PubMed
-
- Sato M., Suzuki S., Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct. Funct. 2003;28:105–112. - PubMed
-
- Blomhoff R., Holte K., Naess L., Berg T. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp. Cell Res. 1984;150:186–193. - PubMed
-
- Bedossa P., Paradis V. Liver extracellular matrix in health and disease. J. Pathol. 2003;200:504–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

