Divergence of the mRNA targets for the Ssb proteins of bacteriophages T4 and RB69
- PMID: 15507125
- PMCID: PMC535899
- DOI: 10.1186/1743-422X-1-4
Divergence of the mRNA targets for the Ssb proteins of bacteriophages T4 and RB69
Abstract
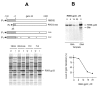
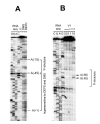
The single-strand binding (Ssb) protein of phage T4 (T4 gp32, product of gene 32) is a mRNA-specific autogenous translational repressor, in addition to being a sequence-independent ssDNA-binding protein that participates in phage DNA replication, repair and recombination. It is not clear how this physiologically essential protein distinguishes between specific RNA and nonspecific nucleic acid targets. Here, we present phylogenetic evidence suggesting that ssDNA and specific RNA bind the same gp32 domain and that plasticity of this domain underlies its ability to configure certain RNA structures for specific binding. We have cloned and characterized gene 32 of phage RB69, a relative of T4 We observed that RB69 gp32 and T4 gp32 have nearly identical ssDNA binding domains, but diverge in their C-terminal domains. In T4 gp32, it is known that the C-terminal domain interacts with the ssDNA-binding domain and with other phage-induced proteins. In translation assays, we show that RB69 gp32 is, like T4 gp32, an autogenous translational repressor. We also show that the natural mRNA targets (translational operators) for the 2 proteins are diverged in sequence from each other and yet can be repressed by either gp32. Results of chemical and RNase sensitivity assays indicate that the gp32 mRNA targets from the 2 related phages have similar structures, but differ in their patterns of contact with the 2 repressors. These and other observations suggest that a range of gp32-RNA binding specificities may evolve in nature due to plasticity of the protein-nucleic acid interaction and its response to modulation by the C-terminal domain of this translational repressor.
Figures


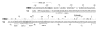

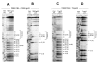
References
-
- Alberts BM, Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970;227:1313–1318. - PubMed
-
- Kreuzer KN, Morrical SW. in Initiation of DNA Replication. Molecular Biology of Bacteriophage T4. 1994. pp. 28–42.
-
- Mosig G. in Recombination Models and Pathways. Molecular Biology of Bacteriophage T4. 1994. pp. 54–82.
-
- Lefebvre SD, Wong ML, Morrical SW. Simultaneous interactions of bacteriophage T4 DNA replication proteins gp59 and gp32 with single-stranded (ss) DNA. Co-modulation of ssDNA binding activities in a DNA helicase assembly intermediate. J Biol Chem. 1999;274:22830–22838. doi: 10.1074/jbc.274.32.22830. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

