Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination
- PMID: 15507306
- PMCID: PMC7112652
- DOI: 10.1016/j.vetimm.2004.09.018
Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination
Abstract
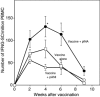
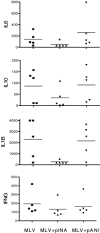
The natural response of pigs to porcine reproductive and respiratory syndrome virus (PRRSV) infections and vaccinations needs to be altered so that better protection is afforded against both homologous and heterologous challenges by this pathogen. To address this problem, real-time gene expression assays were coupled with cytokine Elispot and protein analyses to assess the nature of the anti-PRRSV response of pigs immunized with modified live virus (MLV) vaccine. Although T helper 1 (Th1) immunity was elicited in all vaccinated animals, as evidenced by the genesis of PRRSV-specific interferon-gamma secreting cells (IFNG SC), the overall extent of the memory response was variable and generally weak. Peripheral blood mononuclear cells (PBMC) isolated from these pigs responded to PRRSV exposure with a limited increase in their expression of the Th1 immune markers, IFNG, tumor necrosis factor-alpha and interleukin-15 (IL15), and a reduction in the quantity of mRNAs encoding the innate and inflammatory proteins, IL1B, IL8 and IFNA. Efforts to enhance Th1 immunity, by utilizing an expression plasmid encoding porcine IFNA (pINA) as an adjuvant, resulted in a temporary increase in the frequency of PRRSV-specific IFNG SC but only minor changes overall in the expression of Th1 associated cytokine or innate immune marker mRNA by virus-stimulated PBMC. Administration of pINA, however, did correlate with decreased IL1B secretion by cultured, unstimulated PBMC but had no effect on their ability to release IFNG. Thus, while exogenous addition of IFNA during PRRSV vaccination has an impact on the development of a Th1 immune response, other alterations will be required for substantial boosting of virus-specific protection.
Figures

Similar articles
-
Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection.Vet Immunol Immunopathol. 2013 Feb 15;151(3-4):193-206. doi: 10.1016/j.vetimm.2012.11.006. Epub 2012 Nov 19. Vet Immunol Immunopathol. 2013. PMID: 23228653
-
Recombinant Porcine Reproductive and Respiratory Syndrome Virus Expressing Membrane-Bound Interleukin-15 as an Immunomodulatory Adjuvant Enhances NK and γδ T Cell Responses and Confers Heterologous Protection.J Virol. 2018 Jun 13;92(13):e00007-18. doi: 10.1128/JVI.00007-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29643245 Free PMC article.
-
Co-administration of saponin quil A and PRRSV-1 modified-live virus vaccine up-regulates gene expression of type I interferon-regulated gene, type I and II interferon, and inflammatory cytokines and reduces viremia in response to PRRSV-2 challenge.Vet Immunol Immunopathol. 2018 Nov;205:24-34. doi: 10.1016/j.vetimm.2018.10.005. Epub 2018 Oct 24. Vet Immunol Immunopathol. 2018. PMID: 30458999
-
Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction.Vaccine. 2015 Aug 7;33(33):4069-80. doi: 10.1016/j.vaccine.2015.06.092. Epub 2015 Jul 4. Vaccine. 2015. PMID: 26148878 Review.
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
Cited by
-
Concomitant Swine Influenza A Virus Infection Alters PRRSV1 MLV Viremia in Piglets but Does Not Interfere with Vaccine Protection in Experimental Conditions.Vaccines (Basel). 2021 Apr 7;9(4):356. doi: 10.3390/vaccines9040356. Vaccines (Basel). 2021. PMID: 33917103 Free PMC article.
-
Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs.Can J Vet Res. 2006 Jul;70(3):176-82. Can J Vet Res. 2006. PMID: 16850939 Free PMC article.
-
Fermented rye with Agaricus subrufescens and mannan-rich hydrolysate based feed additive to modulate post-weaning piglet immune response.Porcine Health Manag. 2021 Dec 9;7(1):60. doi: 10.1186/s40813-021-00241-y. Porcine Health Manag. 2021. PMID: 34886904 Free PMC article.
-
Cloning and Expression of the Tibetan Pig Interleukin-23 Gene and Its Promotion of Immunity of Pigs to PCV2 Vaccine.Vaccines (Basel). 2020 May 26;8(2):250. doi: 10.3390/vaccines8020250. Vaccines (Basel). 2020. PMID: 32466622 Free PMC article.
-
ω-3 PUFA rich camelina oil by-products improve the systemic metabolism and spleen cell functions in fattening pigs.PLoS One. 2014 Oct 10;9(10):e110186. doi: 10.1371/journal.pone.0110186. eCollection 2014. PLoS One. 2014. PMID: 25303320 Free PMC article.
References
-
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interf. Cytok. Res. 1998;18:485–490. - PubMed
-
- Asai T., Mori M., Okada M., Uruno K., Yazawa S., Shibata I. Elevated serum haptoglobin in pigs infected with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 1999;70:143–148. - PubMed
-
- Bautista E.M., Molitor T.W. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch. Virol. 1999;144:1191–1200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

