The l2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors
- PMID: 15507604
- PMCID: PMC525100
- DOI: 10.1128/JVI.78.22.12179-12188.2004
The l2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors
Abstract
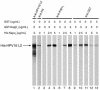
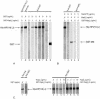
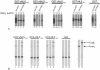
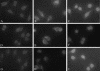
The L2 minor capsid proteins enter the nucleus twice during viral infection: in the initial phase after virion disassembly and in the productive phase when, together with the L1 major capsid proteins, they assemble the replicated viral DNA into virions. In this study we investigated the interactions between the L2 protein of high-risk human papillomavirus type 16 (HPV16) and nuclear import receptors. We discovered that HPV16 L2 interacts directly with both Kapbeta(2) and Kapbeta(3). Moreover, binding of Ran-GTP to either Kapbeta(2) or Kapbeta(3) inhibits its interaction with L2, suggesting that the Kapbeta/L2 complex is import competent. In addition, we found that L2 forms a complex with the Kapalpha(2)beta(1) heterodimer via interaction with the Kapalpha(2) adapter. In agreement with the binding data, nuclear import of L2 in digitonin-permeabilized cells could be mediated by either Kapalpha(2)beta(1) heterodimers, Kapbeta(2), or Kapbeta(3). Mapping studies revealed that HPV16 L2 contains two nuclear localization signals (NLSs), in the N terminus (nNLS) and C terminus (cNLS), that could mediate its nuclear import. Together the data suggest that HPV16 L2 interacts via its NLSs with a network of karyopherins and can enter the nucleus via several import pathways mediated by Kapalpha(2)beta(1) heterodimers, Kapbeta(2), and Kapbeta(3).
Figures






References
-
- Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domains (ND) 10 homing and reorganization. Virology 314:161-167. - PubMed
-
- Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. - PubMed
-
- Chook, Y. M., and G. Blobel. 1999. Structure of the nuclear transport complex karyopherin-β2-Ran x GppNHp. Nature 399:230-237. - PubMed
-
- Coutavas, E., M. Ren, J. D. Oppenheim, P. D'Eustachio, and M. G. Rush. 1993. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature 366:585-587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

