Adenovirus E3-6.7K protein is required in conjunction with the E3-RID protein complex for the internalization and degradation of TRAIL receptor 2
- PMID: 15507617
- PMCID: PMC525093
- DOI: 10.1128/JVI.78.22.12297-12307.2004
Adenovirus E3-6.7K protein is required in conjunction with the E3-RID protein complex for the internalization and degradation of TRAIL receptor 2
Abstract
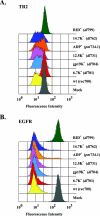
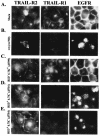
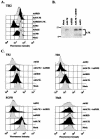
Adenoviruses (Ads) encode several proteins within the early region 3 (E3) transcription unit that help protect infected cells from elimination by the immune system. Among these immunomodulatory proteins, the receptor internalization and degradation (RID) protein complex, which is composed of the RIDalpha (formerly E3-10.4K) and RIDbeta (formerly E3-14.5K) subunits, stimulates the internalization and degradation of certain members of the tumor necrosis factor (TNF) receptor superfamily, thus blocking apoptosis initiated by Fas and TNF-related apoptosis-inducing ligand (TRAIL). The experiments reported here show that TRAIL receptor 2 (TR2) is cleared from the cell surface in Ad-infected cells. Virus mutants containing deletions that span E3 were used to show that the RID and E3-6.7K proteins are both necessary for the internalization and degradation of TR2, whereas only the RID protein is required for TRAIL receptor 1 downregulation. In addition, replication-defective Ad vectors that express individual E3 proteins were used to establish that the RID and E3-6.7K proteins are sufficient to clear TR2. These data demonstrate that E3-6.7K is an important component of the antiapoptosis arsenal encoded by the E3 transcription unit of subgroup C Ads.
Figures




Similar articles
-
Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins.J Virol. 2001 Oct;75(19):8875-87. doi: 10.1128/JVI.75.19.8875-8887.2001. J Virol. 2001. PMID: 11533151 Free PMC article.
-
Construction and characterization of E1-minus replication-defective adenovirus vectors that express E3 proteins from the E1 region.Virology. 2002 Sep 15;301(1):99-108. doi: 10.1006/viro.2002.1580. Virology. 2002. PMID: 12359450
-
Adenovirus RIDbeta subunit contains a tyrosine residue that is critical for RID-mediated receptor internalization and inhibition of Fas- and TRAIL-induced apoptosis.J Virol. 2002 Nov;76(22):11329-42. doi: 10.1128/jvi.76.22.11329-11342.2002. J Virol. 2002. PMID: 12388693 Free PMC article.
-
Functions and mechanisms of action of the adenovirus E3 proteins.Int Rev Immunol. 2004 Jan-Apr;23(1-2):75-111. doi: 10.1080/08830180490265556. Int Rev Immunol. 2004. PMID: 14690856 Review.
-
Subversion of host defense mechanisms by adenoviruses.Curr Top Microbiol Immunol. 2002;269:273-318. doi: 10.1007/978-3-642-59421-2_16. Curr Top Microbiol Immunol. 2002. PMID: 12224514 Review.
Cited by
-
Mechanism for removal of tumor necrosis factor receptor 1 from the cell surface by the adenovirus RIDalpha/beta complex.J Virol. 2005 Nov;79(21):13606-17. doi: 10.1128/JVI.79.21.13606-13617.2005. J Virol. 2005. PMID: 16227281 Free PMC article.
-
Identification of a novel immunosubversion mechanism mediated by a virologue of the B-lymphocyte receptor TACI.Clin Vaccine Immunol. 2007 Jul;14(7):907-17. doi: 10.1128/CVI.00058-07. Epub 2007 May 30. Clin Vaccine Immunol. 2007. PMID: 17538121 Free PMC article.
-
A novel hybrid adenoretroviral vector with more extensive E3 deletion extends transgene expression in submandibular glands.Hum Gene Ther Methods. 2012 Jun;23(3):169-81. doi: 10.1089/hgtb.2011.175. Epub 2012 Jul 20. Hum Gene Ther Methods. 2012. PMID: 22817829 Free PMC article.
-
Adenovirus RIDalpha regulates endosome maturation by mimicking GTP-Rab7.J Cell Biol. 2007 Dec 3;179(5):965-80. doi: 10.1083/jcb.200702187. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039930 Free PMC article.
-
Adenoviromics: Mining the Human Adenovirus Species D Genome.Front Microbiol. 2018 Sep 11;9:2178. doi: 10.3389/fmicb.2018.02178. eCollection 2018. Front Microbiol. 2018. PMID: 30254627 Free PMC article. Review.
References
-
- Benedict, C., P. Norris, T. Prigozy, J. L. Bodmer, J. A. Mahr, C. Garnett, F. Martinon, J. Tschopp, L. R. Gooding, and C. F. Ware. 2001. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and-2. J. Biol. Chem. 276:3270-3278. - PubMed
-
- Benedict, C. A. 2003. Viruses and the TNF-related cytokines, an evolving battle. Cytokine Growth Factor Rev. 14:349-357. - PubMed
-
- Bett, A. J., V. Krougliak, and F. L. Graham. 1995. DNA sequence of the deletion/insertion in early region 3 of Ad5 dl309. Virus Res. 39:75-82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

