Transition from acute to persistent Theiler's virus infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease
- PMID: 15507635
- PMCID: PMC525090
- DOI: 10.1128/JVI.78.22.12480-12488.2004
Transition from acute to persistent Theiler's virus infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease
Abstract
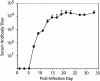
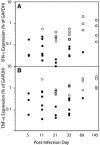
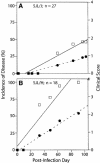
The dynamics of Theiler's murine encephalomyelitis virus (TMEV) RNA replication in the central nervous systems of susceptible and resistant strains of mice were examined by quantitative real-time reverse transcription-PCR and were found to correlate with host immune responses. During the acute phase of infection in both susceptible and resistant mice, levels of viral replication were high in the brain and brain stem, while levels of viral genome equivalents were 10- to 100-fold lower in the spinal cord. In the brain, viral RNA replication decreased after a peak at 5 days postinfection (p.i.), in parallel with the appearance of virus-specific antibody responses; however, by 15 days p.i., viral RNA levels began to increase in the spinal cords of susceptible mice. During the transition to and the persistent phase of infection, the numbers of viral genome equivalents in the spinal cord varied substantially for individual mice, but high levels were consistently associated with high levels of proinflammatory Th1 cytokine and chemokine mRNAs. Moreover, a large number of viral genome equivalents and high proinflammatory cytokine mRNA levels in spinal cords were only observed for susceptible SJL/J mice who developed demyelinating disease. These results suggest that TMEV persistence requires active viral replication beginning about day 11 p.i. and that active viral replication with high viral genome loads leads to increased levels of Th1 cytokines that drive disease progression in infected mice.
Figures




References
-
- Aubert, C., M. Chamorro, and M. Brahic. 1987. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb. Pathog. 3:319-326. - PubMed
-
- Begolka, W. S., C. L. Vanderlugt, S. M. Rahbe, and S. D. Miller. 1999. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J. Immunol. 161:4437-4446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

