Identification of TAZ as a binding partner of the polyomavirus T antigens
- PMID: 15507652
- PMCID: PMC525041
- DOI: 10.1128/JVI.78.22.12657-12664.2004
Identification of TAZ as a binding partner of the polyomavirus T antigens
Abstract
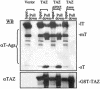
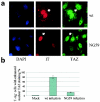
A polyomavirus mutant isolated by the tumor host range selection procedure (19) has a three-amino-acid deletion (Delta2-4) in the common N terminus of the T antigens. To search for a cellular protein bound by wild-type but not the mutant T antigen(s), a yeast two-hybrid screen of a mouse embryo cDNA library was carried out with a bait of wild-type small T antigen (sT) fused N terminally to the DNA-binding domain of Gal4. TAZ, a transcriptional coactivator with a WW domain and PDZ-binding motif (17), was identified as a binding partner. TAZ bound in vivo to all three T antigens with different apparent affinities estimated as 1:7:100 (large T antigen [lT]:middle T antigen [mT]:sT). The Delta2-4 mutant T antigens showed no detectable binding. The sT and mT of the host range transformation-defective (hr-t) mutant NG59 with an alteration in the common sT/mT region (179 D-->NI) and a normal N terminus also failed to bind TAZ, while the unaltered lT bound but with reduced affinity compared to that seen in a wild-type virus infection. The WW domain but not the PDZ-binding motif of TAZ was essential for T antigen binding. The Delta2-4 mutant was defective in viral DNA replication. Forced overexpression of TAZ blocked wild-type DNA replication in a manner dependent on the binding site for the polyomavirus enhancer-binding protein 2alpha. Wild-type polyomavirus T antigens effectively block transactivation by TAZ. The functional significance of TAZ interactions with polyomavirus T antigens is discussed.
Figures




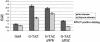
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

