Functional characterization of a small conductance GIRK channel in rat atrial cells
- PMID: 15507689
- PMCID: PMC1304783
- DOI: 10.1529/biophysj.103.039487
Functional characterization of a small conductance GIRK channel in rat atrial cells
Erratum in
- Biophys J. 2005 Jan;88(1):757
Abstract
Muscarinic K+ (KACh) channels are key determinants of the inhibitory synaptic transmission in the heart. These channels are heterotetramers consisting of two homologous subunits, G-protein-gated inwardly rectifying K+ (GIRK)1 and GIRK4, and have unitary conductance of approximately 35 pS with symmetrical 150 mM KCl solutions. Activation of atrial KACh channels, however, is often accompanied by the appearance of openings with a lower conductance, suggesting a functional heterogeneity of G-protein-sensitive ion channels in the heart. Here we report the characterization of a small conductance GIRK (scGIRK) channel present in rat atria. This channel is directly activated by Gbetagamma subunits and has a unitary conductance of 16 pS. The scGIRK and KACh channels display similar affinities for Gbetagamma binding and are frequently found in the same membrane patches. Furthermore, Gbetagamma-activated scGIRK channels--like their KACh counterparts--exhibit complex gating behavior, fluctuating among four functional modes conferred by the apparent binding of a different number of Gbetagamma subunits to the channel. The electrogenic efficacy of the scGIRK channels, however, is negligible compared to that of KACh channels. Thus, Gbetagamma subunits employ the same signaling strategy to regulate two ion channels that are apparently endowed with very different functions in the atrial membrane.
Figures









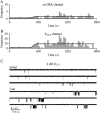
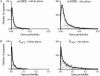


References
-
- Bettahi, I., C. L. Marker, M. I. Roman, and K. Wickman. 2002. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J. Biol. Chem. 277:48282–48288. - PubMed
-
- Colquhoun, D., and A. G. Hawkes. 1981. On the stochastic properties of single ion channels. Proc. R. Soc. B. 211:205–235. - PubMed
-
- Colquhoun, D., and A. G. Hawkes. 1983. The principles of the stochastic interpretation of ion-channel mechanisms. In Single Channel Recording. B. Sakmann, and E. Neher, editors. Plenum Press, New York. 135–175.
-
- Corey, S., and D. E. Clapham. 1998. Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J. Biol. Chem. 273:27499–27504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

