Add-on therapy options in asthma not adequately controlled by inhaled corticosteroids: a comprehensive review
- PMID: 15509300
- PMCID: PMC528858
- DOI: 10.1186/1465-9921-5-17
Add-on therapy options in asthma not adequately controlled by inhaled corticosteroids: a comprehensive review
Abstract
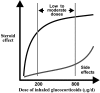
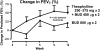
Many patients with persistent asthma can be controlled with inhaled corticosteroids (ICS). However, a considerable proportion of patients remain symptomatic, despite the use of ICS. We present systematically evidence that supports the different treatment options. A literature search was made of Medline/PubMed to identify randomised and blinded trials. To demonstrate the benefit that can be obtained by increasing the dose of ICS, dose-response studies with at least three different ICS doses were identified. To demonstrate whether more benefit can be obtained by adding long-acting beta2-agonist (LABA), leukotriene antagonist (LTRA) or theophylline than by increasing the dose of ICS, studies comparing these options were identified. Thirdly, studies comparing the different "add-on" options were identified. The addition of a LABA is more effective than increasing the dose of ICS in improving asthma control. By increasing the dose of ICS, clinical improvement is likely to be of small magnitude. Addition of a LTRA or theophylline to the treatment regimen appears to be equivalent to doubling the dose of ICS. Addition of a LABA seems to be superior to an LTRA in improving lung function. However, addition of LABA and LTRA may be equal with respect to asthma exacerbations. However, more and longer studies are needed to better clarify the role of LTRAs and theophylline as add-on therapies.
Figures
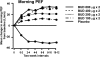
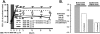
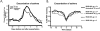

References
-
- NHLBI. National Asthma Education and Prevention Program, Expert Panel Report 2 . NIH Publication No 97-4051. Bethesda, MD: US Department of Health and Human Services; 1997. Guidelines for the diagnosis and management of asthma.
-
- NHLBI. Global Initiative for Asthma Global strategy for astma management and prevention. NIH Publication No 02-3659. 2002.
-
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S1–S53. - PubMed
-
- Martin RJ, Szefler SJ, Chincilli VM, Kraft M, Dolovich M, Boushey HA, Cherniak RM, Craig TJ, Drazen JM, Fagan JK, Fahy JV, Fish JE, Ford JG, Israel E, Kunselman SJ, Lazarus SC, Lemanske RF, Jr, Peters SP, Sorkness CA. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165:1377–1383. - PubMed
-
- The British Thoracic Society The British guidelines on asthma management 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

