T-cell properties determine disease site, clinical presentation, and cellular pathology of experimental autoimmune encephalomyelitis
- PMID: 15509523
- PMCID: PMC1618652
- DOI: 10.1016/S0002-9440(10)63410-4
T-cell properties determine disease site, clinical presentation, and cellular pathology of experimental autoimmune encephalomyelitis
Abstract
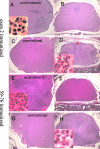
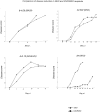
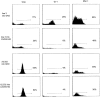
Two distinct clinical phenotypes of experimental autoimmune encephalomyelitis are observed in BALB interferon-gamma knockout mice immunized with encephalitogenic peptides of myelin basic protein. Conventional disease, characterized by ascending weakness and paralysis, occurs with greater frequency after immunizing with a peptide comprising residues 59 to 76. Axial-rotatory disease, characterized by uncontrolled axial rotation, occurs with greater frequency in mice immunized with a peptide corresponding to exon 2 of the full length 21.5-kd protein. The two clinical phenotypes are histologically distinguishable. Conventional disease is characterized by inflammation and demyelination primarily in spinal cord, whereas axial-rotatory disease involves inflammation and demyelination of lateral medullary areas of brain. Both types have infiltrates in which neutrophils are a predominating component. By isolating T cells and transferring disease to naive recipients, we show here that the type of disease is determined entirely by the inducing T cell. Furthermore, studies using CXCR2 knockout recipients, unable to recruit neutrophils to inflammatory sites, show that although neutrophils are critical for some of these T cells to effect disease, there are also interferon-gamma-deficient T cells that induce disease in the absence of both interferon-gamma and neutrophils. These results highlight the multiplicity of T-cell-initiated effector pathways available for inflammation and demyelination.
Figures





References
-
- Abromson-Leeman S, Hayashi M, Martin C, Sobel R, al-Sabbagh A, Weiner H, Dorf ME. T cell responses to myelin basic protein in experimental autoimmune encephalomyelitis-resistant BALB/c mice. J Neuroimmunol. 1993;45:89–101. - PubMed
-
- Abromson-Leeman S, Alexander J, Bronson R, Carroll J, Southwood S, Dorf M. Experimental autoimmune encephalomyelitis-resistant mice have highly encephalitogenic myelin basic protein (MBP)-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J Immunol. 1995;154:388–398. - PubMed
-
- Yoshizawa I, Bronson R, Ben-Nun A, Richert JR, Dorf ME, Abromson-Leeman S. Differential recognition of MBP epitopes in BALB/c mice determines the site of inflammatory disease induction. J Neuroimmunol. 1998;89:73–82. - PubMed
-
- Yoshizawa I, Bronson R, Dorf ME, Abromson-Leeman S. T-cell responses to myelin basic protein in normal and MBP-deficient mice. J Neuroimmunol. 1998;84:131–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

