Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts
- PMID: 15509552
- PMCID: PMC1618653
- DOI: 10.1016/S0002-9440(10)63439-6
Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts
Abstract
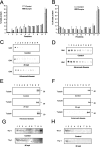
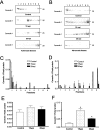
The main event in the pathogenesis of prion diseases is the conversion of the cellular prion protein (PrP(C)) into the abnormal, protease-resistant prion protein (PrP(res)). PrP(C) is a GPI-anchored protein located in lipid rafts or detergent-resistant membranes (DRMs). Here we describe the association of PrP with DRMs in neuronal cell bodies and axons during the course of murine scrapie and its relation with the distribution of the PrP-interacting proteins caveolin 1 and synaptophysin. Scrapie infection triggered the accumulation of PrP(res) in DRMs from retinas and optic nerves from early stages of the disease before evidence of neuronal cell loss. Most of the PrP(res) remained associated with lipid rafts throughout different stages in disease progression. In contrast to PrP(res), caveolin 1 and synaptophysin in retina and optic nerves shifted to non-DRM fractions during the course of scrapie infection. The accumulation of PrP(res) in DRMs was not associated with a general alteration in their composition, because no change in the total protein distribution across the sucrose gradient or in the flotation characteristics of the glycosphingolipid GM1 or Thy-1 were observed until advanced stages of the disease. However, an increase in total cholesterol levels was observed in optic nerve and retinas. Only during late stages of the disease was a decrease in the number of neuronal cell bodies observed, suggesting that synaptic abnormalities are the earliest sign of neuronal dysfunction that ultimately results in neuronal death. These results indicate that prion replication triggers an abnormal localization of caveolin 1 and synaptophysin, which in turn may alter neuronal function.
Figures





Similar articles
-
Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin.J Neurochem. 2005 Sep;94(6):1696-710. doi: 10.1111/j.1471-4159.2005.03331.x. Epub 2005 Jul 25. J Neurochem. 2005. PMID: 16045452
-
The prion protein requires cholesterol for cell surface localization.Mol Cell Neurosci. 2006 Feb;31(2):346-53. doi: 10.1016/j.mcn.2005.10.008. Epub 2005 Nov 8. Mol Cell Neurosci. 2006. PMID: 16278084
-
Isolation at physiological temperature of detergent-resistant membranes with properties expected of lipid rafts: the influence of buffer composition.Biochem J. 2009 Jan 15;417(2):525-33. doi: 10.1042/BJ20081385. Biochem J. 2009. PMID: 18831713
-
Glypican-1 facilitates prion conversion in lipid rafts.J Neurochem. 2011 Mar;116(5):721-5. doi: 10.1111/j.1471-4159.2010.06936.x. J Neurochem. 2011. PMID: 20681952 Review.
-
Cellular prion protein signaling in serotonergic neuronal cells.Ann N Y Acad Sci. 2007 Jan;1096:106-19. doi: 10.1196/annals.1397.076. Ann N Y Acad Sci. 2007. PMID: 17405922 Review.
Cited by
-
Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth.J Cell Sci. 2009 Jan 15;122(Pt 2):289-99. doi: 10.1242/jcs.030338. J Cell Sci. 2009. PMID: 19118221 Free PMC article.
-
Do prion protein gene polymorphisms induce apoptosis in non-mammals?J Biosci. 2016 Mar;41(1):97-107. doi: 10.1007/s12038-015-9584-7. J Biosci. 2016. PMID: 26949092
-
Reversibility of prion-induced neurodegeneration.Lancet Neurol. 2007 Apr;6(4):294-5. doi: 10.1016/S1474-4422(07)70064-9. Lancet Neurol. 2007. PMID: 17362830 Free PMC article. No abstract available.
-
REST alleviates neurotoxic prion peptide-induced synaptic abnormalities, neurofibrillary degeneration and neuronal death partially via LRP6-mediated Wnt-β-catenin signaling.Oncotarget. 2016 Mar 15;7(11):12035-52. doi: 10.18632/oncotarget.7640. Oncotarget. 2016. PMID: 26919115 Free PMC article.
-
Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra.Biochemistry. 2011 May 31;50(21):4479-90. doi: 10.1021/bi2003907. Epub 2011 May 3. Biochemistry. 2011. PMID: 21539311 Free PMC article.
References
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Johnson RT, Gibbs CJ., Jr Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994–2004. - PubMed
-
- Borchelt DR, Taraboulos A, Prusiner SB. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992;267:16188–16199. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

