Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala
- PMID: 15509737
- PMCID: PMC6730154
- DOI: 10.1523/JNEUROSCI.3567-04.2004
Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala
Abstract
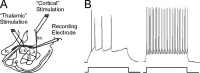
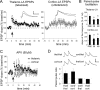
Long-term potentiation (LTP) of synaptic transmission in the lateral amygdala (LA) is believed to underlie the formation and retention of fear memories. To explore the role of inhibitory transmission in amygdala plasticity, we recorded from LA inhibitory interneurons in vitro before and after tetanization of the thalamo-LA pathway, one of the major inputs to LA involved in fear learning. Tetanization resulted in LTP of the EPSPs elicited in both the tetanized thalamic pathway and the untetanized cortical pathway to LA. This LTP was NMDA-dependent and associated with a decrease in paired-pulse facilitation in both pathways. In LA excitatory cells, LTP of interneurons resulted in an increase in the amplitude of GABAergic IPSPs in both input pathways. Finally, isolated GABAergic IPSPs between inhibitory and excitatory neurons could be potentiated as well. Plasticity of inhibitory transmission within the LA may therefore contribute significantly to LA-mediated functions, such as fear conditioning.
Figures


Similar articles
-
Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways.Neuron. 2006 Dec 7;52(5):883-96. doi: 10.1016/j.neuron.2006.10.010. Neuron. 2006. PMID: 17145508 Free PMC article.
-
Long-term potentiation in freely moving rats reveals asymmetries in thalamic and cortical inputs to the lateral amygdala.Eur J Neurosci. 2003 Jun;17(12):2703-15. doi: 10.1046/j.1460-9568.2003.02707.x. Eur J Neurosci. 2003. PMID: 12823477
-
A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala.J Neurosci. 2010 Nov 3;30(44):14619-29. doi: 10.1523/JNEUROSCI.3252-10.2010. J Neurosci. 2010. PMID: 21048119 Free PMC article.
-
GABAergic interneurons: The orchestra or the conductor in fear learning and memory?Brain Res Bull. 2018 Jul;141:13-19. doi: 10.1016/j.brainresbull.2017.11.016. Epub 2017 Dec 2. Brain Res Bull. 2018. PMID: 29197563 Free PMC article. Review.
-
GABA through the ages: regulation of cortical function and plasticity by inhibitory interneurons.Neural Plast. 2012;2012:892784. doi: 10.1155/2012/892784. Epub 2012 Jun 26. Neural Plast. 2012. PMID: 22792496 Free PMC article. Review.
Cited by
-
Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity.Brain Res. 2008 Mar 20;1200:58-65. doi: 10.1016/j.brainres.2008.01.057. Epub 2008 Feb 2. Brain Res. 2008. PMID: 18295751 Free PMC article.
-
Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II.Neuron. 2009 Feb 12;61(3):351-8. doi: 10.1016/j.neuron.2008.12.030. Neuron. 2009. PMID: 19217373 Free PMC article.
-
Arc expression identifies the lateral amygdala fear memory trace.Mol Psychiatry. 2016 Mar;21(3):364-75. doi: 10.1038/mp.2015.18. Epub 2015 Mar 24. Mol Psychiatry. 2016. PMID: 25802982 Free PMC article.
-
Synaptic maturation at cortical projections to the lateral amygdala in a mouse model of Rett syndrome.PLoS One. 2010 Jul 2;5(7):e11399. doi: 10.1371/journal.pone.0011399. PLoS One. 2010. PMID: 20625482 Free PMC article.
-
Olfactory threat extinction in the piriform cortex: An age-dependent employment of NMDA receptor-dependent long-term depression.Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2309986120. doi: 10.1073/pnas.2309986120. Epub 2023 Oct 25. Proc Natl Acad Sci U S A. 2023. PMID: 37878718 Free PMC article.
References
-
- Chapman PF, Kairiss EW, Keenan CL, Brown TH (1990) Long-term synaptic potentiation in the amygdala. Synapse 6: 271-278. - PubMed
-
- Doyere V, Schafe GE, Sigurdsson T, LeDoux JE (2003) Long-term potentiation in freely moving rats reveals asymmetries in thalamic and cortical inputs to the lateral amygdala. Eur J Neurosci 17: 2703-2715. - PubMed
-
- Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6: 347-470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
