Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations
- PMID: 15509753
- PMCID: PMC6730151
- DOI: 10.1523/JNEUROSCI.2973-04.2004
Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations
Abstract
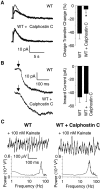
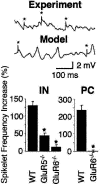
Kainate receptors (KARs) play an important role in synaptic physiology, plasticity, and pathological phenomena such as epilepsy. However, the physiological implications for neuronal networks of the distinct expression patterns of KAR subunits are unknown. Using KAR knock-out mice, we show that subunits glutamate receptor (GluR) 5 and GluR6 play distinct roles in kainate-induced gamma oscillations and epileptiform burst activity. Ablation of GluR5 leads to a higher susceptibility of the network to the oscillogenic and epileptogenic effects of kainate, whereas lack of GluR6 prevents kainate-induced gamma oscillations or epileptiform bursts. Based on experimental and simulated neuronal network data as well as the consequences of GluR5 and GluR6 expression for cellular and synaptic physiology, we propose that the functional interplay of GluR5-containing KARs on axons of interneurons and GluR6-containing KARs in the somatodendritic region of both interneurons and pyramidal cells underlie the oscillogenic and epileptogenic effects of kainate.
Figures
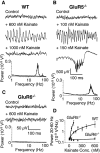

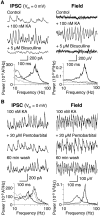

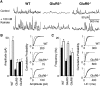
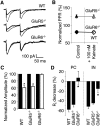
References
-
- Ben-Ari Y (1985) Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14: 375-403. - PubMed
-
- Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci 23: 580-587. - PubMed
-
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL (1999) Kainate receptors are involved in synaptic plasticity. Nature 402: 297-301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
