The TBC (Tre-2/Bub2/Cdc16) domain protein TRE17 regulates plasma membrane-endosomal trafficking through activation of Arf6
- PMID: 15509780
- PMCID: PMC525471
- DOI: 10.1128/MCB.24.22.9752-9762.2004
The TBC (Tre-2/Bub2/Cdc16) domain protein TRE17 regulates plasma membrane-endosomal trafficking through activation of Arf6
Abstract
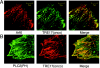
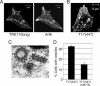
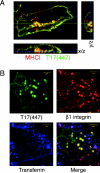
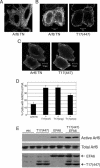
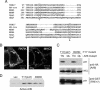
TBC (Tre-2/Bub2/Cdc16) domains are predicted to encode GTPase-activating proteins (GAPs) for Rab family G proteins. While approximately 50 TBC proteins are predicted to exist in humans, little is known about their substrate specificity. Here we show that TRE17 (also called Tre-2 and USP6), a founding member of the TBC family, targets the Arf family GTPase Arf6, which regulates plasma membrane-endosome trafficking. Surprisingly, TRE17 does not function as a GAP for Arf6 but rather promotes its activation in vivo. TRE17 associates directly with Arf6 in its GDP- but not GTP-bound state. Mapping experiments pinpoint the site of interaction to the TBC domain of TRE17. Forced expression of TRE17 promotes the localization of Arf6 to the plasma membrane, leading to Arf6 activation, presumably due to facilitated access to membrane-associated guanine nucleotide exchange factors (GEFs). Furthermore, TRE17 cooperates with Arf6 GEFs to induce GTP loading of Arf6 in vivo. Finally, short interfering RNA-mediated loss of TRE17 leads to attenuated Arf6 activation. These studies identify TRE17 as a novel regulator of the Arf6-regulated plasma membrane recycling system and reveal an unexpected function for TBC domains.
Figures


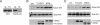

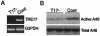
Similar articles
-
The oncogenic TBC domain protein USP6/TRE17 regulates cell migration and cytokinesis.Biol Cell. 2012 Jan;104(1):22-33. doi: 10.1111/boc.201100108. Epub 2011 Dec 1. Biol Cell. 2012. PMID: 22188517
-
Calcium/calmodulin regulates ubiquitination of the ubiquitin-specific protease TRE17/USP6.J Biol Chem. 2005 Oct 28;280(43):35967-73. doi: 10.1074/jbc.M505220200. Epub 2005 Aug 26. J Biol Chem. 2005. PMID: 16127172
-
TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation.J Biol Chem. 2010 Nov 19;285(47):37111-20. doi: 10.1074/jbc.M110.175133. Epub 2010 Sep 23. J Biol Chem. 2010. PMID: 20864534 Free PMC article.
-
The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma.Front Biosci (Schol Ed). 2012 Jan 1;4(1):321-34. doi: 10.2741/s271. Front Biosci (Schol Ed). 2012. PMID: 22202063 Review.
-
TBC proteins: GAPs for mammalian small GTPase Rab?Biosci Rep. 2011 Jun;31(3):159-68. doi: 10.1042/BSR20100112. Biosci Rep. 2011. PMID: 21250943 Review.
Cited by
-
A-RAF kinase functions in ARF6 regulated endocytic membrane traffic.PLoS One. 2009;4(2):e4647. doi: 10.1371/journal.pone.0004647. Epub 2009 Feb 27. PLoS One. 2009. PMID: 19247477 Free PMC article.
-
Regulation of the Hippo signaling pathway by deubiquitinating enzymes in cancer.Genes Dis. 2019 Jun 24;6(4):335-341. doi: 10.1016/j.gendis.2019.06.004. eCollection 2019 Dec. Genes Dis. 2019. PMID: 31832513 Free PMC article. Review.
-
Jak1-STAT3 Signals Are Essential Effectors of the USP6/TRE17 Oncogene in Tumorigenesis.Cancer Res. 2016 Sep 15;76(18):5337-47. doi: 10.1158/0008-5472.CAN-15-2391. Epub 2016 Jul 20. Cancer Res. 2016. PMID: 27440725 Free PMC article.
-
USP6 activation in nodular fasciitis by promoter-swapping gene fusions.Mod Pathol. 2017 Nov;30(11):1577-1588. doi: 10.1038/modpathol.2017.78. Epub 2017 Jul 28. Mod Pathol. 2017. PMID: 28752842
-
Comparison of Human Tissue Microarray to Human Pericyte Transcriptome Yields Novel Perivascular Cell Markers.Stem Cells Dev. 2019 Sep 15;28(18):1214-1223. doi: 10.1089/scd.2019.0106. Epub 2019 Aug 1. Stem Cells Dev. 2019. PMID: 31264500 Free PMC article.
References
-
- Albert, S., and D. Gallwitz. 1999. Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem. 274:33186-33189. - PubMed
-
- Albert, S., and D. Gallwitz. 2000. Msb4p, a protein involved in Cdc42p-dependent organization of the actin cytoskeleton, is a Ypt/Rab-specific GAP. Biol. Chem. 381:453-456. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Curr. Prot. Mol. Biol. II 16.16.11-16.16.15.
-
- Bernards, A. 2003. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603:47-82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
