Tankyrase polymerization is controlled by its sterile alpha motif and poly(ADP-ribose) polymerase domains
- PMID: 15509784
- PMCID: PMC525485
- DOI: 10.1128/MCB.24.22.9802-9812.2004
Tankyrase polymerization is controlled by its sterile alpha motif and poly(ADP-ribose) polymerase domains
Abstract
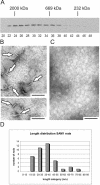
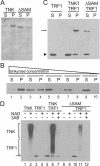
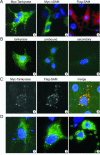
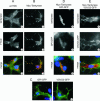
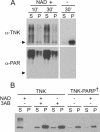
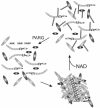
Tankyrases are novel poly(ADP-ribose) polymerases that have SAM and ankyrin protein-interaction domains. They are found at telomeres, centrosomes, nuclear pores, and Golgi vesicles and have been shown to participate in telomere length regulation. Their other function(s) are unknown, and it has been difficult to envision a common role at such diverse cellular locations. We have shown that tankyrase 1 polymerizes through its sterile alpha motif (SAM) domain to assemble large protein complexes. In vitro polymerization is reversible and still allows interaction with ankyrin-domain binding proteins. Polymerization can also occur in vivo, with SAM-dependent association of overexpressed tankyrase leading to formation of large tankyrase-containing vesicles, disruption of Golgi structure, and inhibition of apical secretion. Finally, tankyrase polymers are dissociated efficiently by poly(ADP-ribosy)lation. This disassembly is prevented by mutation of the PARP domain. Our findings indicate that tankyrase 1 has the unique capacity to promote both assembly and disassembly of large protein complexes. Thus, tankyrases appear to be master scaffolding proteins that regulate the formation of dynamic protein networks at different cellular locations. This implies a common scaffolding function for tankyrases at each location, with specific tankyrase interaction partners conferring location-specific roles to each network, e.g., telomere compaction or regulation of vesicle trafficking.
Figures

References
-
- Bae, J., J. R. Donigian, and A. J. Hsueh. 2003. Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J. Biol. Chem. 278:5195-5204. - PubMed
-
- Bennett, V., and A. J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353-1392. - PubMed
-
- Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. - PubMed
-
- Boeckers, T. M., J. Bockmann, M. R. Kreutz, and E. D. Gundelfinger. 2002. ProSAP/Shank proteins-a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 81:903-910. - PubMed
-
- Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3:267-277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
