Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice
- PMID: 15509788
- PMCID: PMC525490
- DOI: 10.1128/MCB.24.22.9848-9862.2004
Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice
Abstract
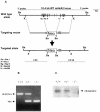
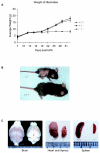
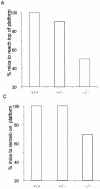
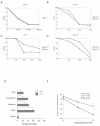
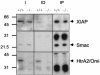
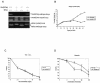
The serine protease HtrA2/Omi is released from the mitochondrial intermembrane space following apoptotic stimuli. Once in the cytosol, HtrA2/Omi has been implicated in promoting cell death by binding to inhibitor of apoptosis proteins (IAPs) via its amino-terminal Reaper-related motif, thus inducing caspase activity, and also in mediating caspase-independent death through its own protease activity. We report here the phenotype of mice entirely lacking expression of HtrA2/Omi due to targeted deletion of its gene, Prss25. These animals, or cells derived from them, show no evidence of reduced rates of cell death but on the contrary suffer loss of a population of neurons in the striatum, resulting in a neurodegenerative disorder with a parkinsonian phenotype that leads to death of the mice around 30 days after birth. The phenotype of these mice suggests that it is the protease function of this protein and not its IAP binding motif that is critical. This conclusion is reinforced by the finding that simultaneous deletion of the other major IAP binding protein, Smac/DIABLO, does not obviously alter the phenotype of HtrA2/Omi knockout mice or cells derived from them. Mammalian HtrA2/Omi is therefore likely to function in vivo in a manner similar to that of its bacterial homologues DegS and DegP, which are involved in protection against cell stress, and not like the proapoptotic Reaper family proteins in Drosophila melanogaster.
Figures




References
-
- Darzynkiewicz, Z., and E. Bedner. 2000. Analysis of apoptotic cells by flow and laser scanning cytometry. Methods Enzymol. 322:18-39. - PubMed
-
- Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. - PubMed
-
- Faccio, L., C. Fusco, A. Chen, S. Martinotti, J. V. Bonventre, and A. S. Zervos. 2000. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J. Biol. Chem. 275:2581-2588. - PubMed
-
- Ghadially, F. N. 1982. Ultrastructural pathology of the cell and matrix, 2nd ed. Butterworths, London, United Kingdom.
-
- Gray, C. W., R. V. Ward, E. Karran, S. Turconi, A. Rowles, D. Viglienghi, C. Southan, A. Barton, K. G. Fantom, A. West, J. Savopoulos, N. J. Hassan, H. Clinkenbeard, C. Hanning, B. Amegadzie, J. B. Davis, C. Dingwall, G. P. Livi, and C. L. Creasy. 2000. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur. J. Biochem. 267:5699-5710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
