The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome
- PMID: 15509792
- PMCID: PMC525476
- DOI: 10.1128/MCB.24.22.9899-9910.2004
The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome
Abstract
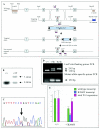
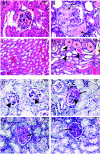
Renal failure is a frequent and costly complication of many chronic diseases, including diabetes and hypertension. One common feature of renal failure is glomerulosclerosis, the pathobiology of which is unclear. To help elucidate this, we generated a mouse strain carrying the missense mutation Wt1 R394W, which predisposes humans to glomerulosclerosis and early-onset renal failure (Denys-Drash syndrome [DDS]). Kidney development was normal in Wt1(+/R394W) heterozygotes. However, by 4 months of age 100% of male heterozygotes displayed proteinuria and glomerulosclerosis characteristic of DDS patients. This phenotype was observed in an MF1 background but not in a mixed B6/129 background, suggestive of the action of a strain-specific modifying gene(s). WT1 encodes a nuclear transcription factor, and the R394W mutation is known to impair this function. Therefore, to investigate the mechanism of Wt1 R394W-induced renal failure, the expression of genes whose deletion leads to glomerulosclerosis (NPHS1, NPHS2, and CD2AP) was quantitated. In mutant kidneys, NPHS1 and NPHS2 were only moderately downregulated (25 to 30%) at birth but not at 2 or 4 months. Expression of CD2AP was not changed at birth but was significantly upregulated at 2 and 4 months. Podocalyxin was downregulated by 20% in newborn kidneys but not in kidneys at later ages. Two other genes implicated in glomerulosclerosis, TGFB1 and IGF1, were upregulated at 2 months and at 2 and 4 months, respectively. It is not clear whether the significant alterations in gene expression are a cause or a consequence of the disease process. However, the data do suggest that Wt1 R394W-induced glomerulosclerosis may be independent of downregulation of the genes for NPHS1, NPHS2, CD2AP, and podocalyxin and may involve other genes yet to be implicated in renal failure. The Wt1(R394W) mouse recapitulates the pathology and disease progression observed in patients carrying the same mutation, and the mutation is completely penetrant in male animals. Thus, it will be a powerful and biologically relevant model for investigating the pathobiology of the earliest events in glomerulosclerosis.
Figures




References
-
- Armstrong, J. F., K. Pritchard-Jones, W. A. Bickmore, N. D. Hastie, and J. B. Bard. 1993. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 40:85-97. - PubMed
-
- Boute, N., O. Gribouval, S. Roselli, F. Benessy, H. Lee, A. Fuchshuber, K. Dahan, M.-C. Gubler, P. Niaudet, and C. Antignac. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24:349-354. - PubMed
-
- Bruening, W., N. Bardeesy, B. L. Silverman, R. A. Cohn, G. A. Machin, A. J. Aronson, D. Housman, and J. Pelletier. 1992. Germline intronic and exonic mutations in the Wilms' tumour gene (WT1) affecting urogenital development. Nat. Genet. 1:144-148. - PubMed
-
- Bruijn, J. A., A. Roos, B. de Geus, and E. de Heer. 1994. Transforming growth factor-beta and the glomerular extracellular matrix in renal pathology. J. Lab. Clin. Med. 123:34-47. - PubMed
-
- Call, K. M., T. Glaser, C. Y. Ito, A. J. Buckler, J. Pelletier, D. A. Haber, E. A. Rose, A. Kral, H. Yeger, W. H. Lewis, C. Jones, and D. E. Housman. 1990. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60:509-520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
