Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2
- PMID: 15509800
- PMCID: PMC525462
- DOI: 10.1128/MCB.24.22.9986-9999.2004
Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2
Abstract
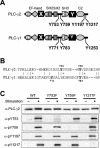
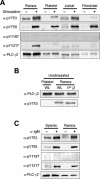
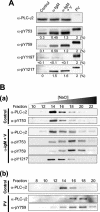
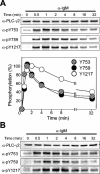
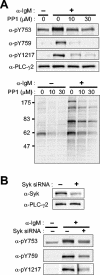
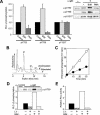
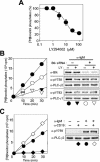
Phospholipase C-gamma2 (PLC-gamma2) plays an important role in B-cell signaling. Phosphorylation of various tyrosine residues of PLC-gamma2 has been implicated in regulation of its lipase activity. With the use of antibodies specific for each of the putative phosphorylation sites, we have now shown that PLC-gamma2 is phosphorylated on Y753, Y759, and Y1217 in response to engagement of the B-cell receptor in Ramos cells, as well as in murine splenic B cells. In cells stimulated maximally via this receptor, the extent of phosphorylation of Y1217 was three times that of Y753 or of Y759. Stimulation of Jurkat T cells or platelets via their immunoreceptors also elicited phosphorylation of Y753 and Y759 but not that of Y1217. A basal level of phosphorylation of Y753 was apparent in unstimulated lymphocytes. The extent of phosphorylation of Y753 and Y759, but not that of Y1217, correlated with the lipase activity of PLC-gamma2. Examination of the effects of various pharmacological inhibitors and of RNA interference in Ramos cells suggested that Btk is largely, but not completely, responsible for phosphorylation of Y753 and Y759, whereas phosphorylation of Y1217 is independent of Btk. Finally, phosphorylation of Y1217 and that of Y753 and Y759 occurred on different PLC-gamma2 molecules.
Figures

References
-
- Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. - PubMed
-
- Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, N. Engels, J. Wienands, L. Simeoni, J. Osterreicher, E. Aguado, M. Malissen, B. Schraven, and V. Horejsi. 2002. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617-1626. - PMC - PubMed
-
- Buhl, A. M., and J. C. Cambier. 1999. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton's tyrosine kinase. J. Immunol. 162:4438-4446. - PubMed
-
- Carpenter, G., and Q. Ji. 1999. Phospholipase C-γ as a signal-transducing element. Exp. Cell Res. 253:15-24. - PubMed
-
- Carter, R. H., D. A. Tuveson, D. J. Park, S. G. Rhee, and D. T. Fearon. 1991. The CD19 complex of B lymphocytes. Activation of phospholipase C by a protein tyrosine kinase-dependent pathway that can be enhanced by the membrane IgM complex. J. Immunol. 147:3663-3671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
