Extracellular signal-regulated kinase 1c (ERK1c), a novel 42-kilodalton ERK, demonstrates unique modes of regulation, localization, and function
- PMID: 15509801
- PMCID: PMC525466
- DOI: 10.1128/MCB.24.22.10000-10015.2004
Extracellular signal-regulated kinase 1c (ERK1c), a novel 42-kilodalton ERK, demonstrates unique modes of regulation, localization, and function
Erratum in
-
Correction for Aebersold et al., "Extracellular Signal-Regulated Kinase 1c (ERK1c), a Novel 42-Kilodalton ERK, Demonstrates Unique Modes of Regulation, Localization, and Function".Mol Cell Biol. 2017 Aug 28;37(18):e00322-17. doi: 10.1128/MCB.00322-17. Print 2017 Sep 15. Mol Cell Biol. 2017. PMID: 28847972 Free PMC article. No abstract available.
Abstract
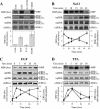
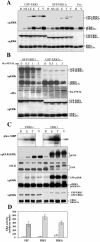
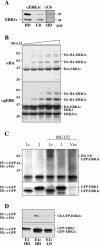
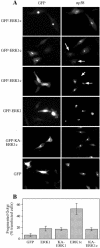
Extracellular signal-regulated kinases (ERKs) are signaling molecules that regulate many cellular processes. We have previously identified an alternatively spliced 46-kDa form of ERK1 that is expressed in rats and mice and named ERK1b. Here we report that the same splicing event in humans and monkeys causes, due to sequence differences in the inserted introns, the production of an ERK isoform that migrates together with the 42-kDa ERK2. Because of the differences of this isoform from ERK1b, we named it ERK1c. We found that its expression levels are about 10% of ERK1. ERK1c seems to be expressed in a wide variety of tissues and cells. Its activation by MEKs and inactivation by phosphatases are slower than those of ERK1, which is probably the reason for its differential regulation in response to extracellular stimuli. Unlike ERK1, ERK1c undergoes monoubiquitination, which is increased with elevated cell density concomitantly with accumulation of ERK1c in the Golgi apparatus. Elevated cell density also causes enhanced Golgi fragmentation, which is facilitated by overexpression of native ERK1c and is prevented by dominant-negative ERK1c, indicating that ERK1c mediates cell density-induced Golgi fragmentation. The differential regulation of ERK1c extends the signaling specificity of MEKs after stimulation by various extracellular stimuli.
Figures





References
-
- Acharya, U., A. Mallabiabarrena, J. K. Acharya, and V. Malhotra. 1998. Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92:183-192. - PubMed
-
- Bott, C. M., S. G. Thorneycroft, and C. J. Marshall. 1994. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 352:201-205. - PubMed
-
- Boucher, M. J., D. Jean, A. Vezina, and N. Rivard. 2004. Dual role of MEK/ERK signaling in senescence and transformation of intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G736-G746. (First published 30 December 2003; http://ajpgi.physiology.org/cgi/content/full/286/5/G736.) - PubMed
-
- Boulton, T. G., S. H. Nye, D. J. Robbins, N. Y. Ip, E. Radziejewska, S. D. Morgenbesser, R. A. DePinho, N. Panayotatos, M. H. Cobb, and G. D. Yancopoulos. 1991. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663-675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
