Application of locked nucleic acids to improve aptamer in vivo stability and targeting function
- PMID: 15509871
- PMCID: PMC528785
- DOI: 10.1093/nar/gkh862
Application of locked nucleic acids to improve aptamer in vivo stability and targeting function
Abstract
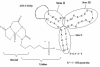
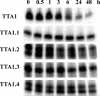
Aptamers are powerful candidates for molecular imaging applications due to a number of attractive features, including rapid blood clearance and tumor penetration. We carried out structure-activity relationship (SAR) studies with the Tenascin-C binding aptamer TTA1, which is a promising candidate for application in tumor imaging with radioisotopes. The aim was to improve its in vivo stability and target binding. We investigated the effect of thermal stabilization of the presumed non-binding double-stranded stem region on binding affinity and resistance against nucleolytic degradation. To achieve maximal thermal stem stabilization melting experiments with model hexanucleotide duplexes consisting of unmodified RNA, 2'-O-methyl RNA (2'-OMe), 2'-Fluoro RNA (2'-F) or Locked Nucleic Acids (LNAs) were initially carried out. Extremely high melting temperatures have been found for an LNA/LNA duplex. TTA1 derivatives with LNA and 2'-OMe modifications within the non-binding stem have subsequently been synthesized. Especially, the LNA-modified TTA1 derivative exhibited significant stem stabilization and markedly improved plasma stability while maintaining its binding affinity to the target. In addition, higher tumor uptake and longer blood retention was found in tumor-bearing nude mice. Thus, our strategy to introduce LNA modifications after the selection procedure is likely to be generally applicable to improve the in vivo stability of aptamers without compromising their binding properties.
Figures




Similar articles
-
Properties of an LNA-modified ricin RNA aptamer.Biochem Biophys Res Commun. 2012 Mar 2;419(1):60-5. doi: 10.1016/j.bbrc.2012.01.127. Epub 2012 Feb 3. Biochem Biophys Res Commun. 2012. PMID: 22326915
-
Carba-LNA-5MeC/A/G/T modified oligos show nucleobase-specific modulation of 3'-exonuclease activity, thermodynamic stability, RNA selectivity, and RNase H elicitation: synthesis and biochemistry.J Org Chem. 2011 Jun 3;76(11):4408-31. doi: 10.1021/jo200073q. Epub 2011 May 4. J Org Chem. 2011. PMID: 21500818
-
Free-radical ring closure to conformationally locked α-L-carba-LNAs and synthesis of their oligos: nuclease stability, target RNA specificity, and elicitation of RNase H.J Org Chem. 2010 Sep 17;75(18):6122-40. doi: 10.1021/jo100900v. J Org Chem. 2010. PMID: 20738147
-
Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals.Chem Soc Rev. 2011 Dec;40(12):5680-9. doi: 10.1039/c1cs15048k. Epub 2011 May 10. Chem Soc Rev. 2011. PMID: 21556437 Review.
-
Locked nucleic acids: promising nucleic acid analogs for therapeutic applications.Chem Biodivers. 2010 Mar;7(3):536-42. doi: 10.1002/cbdv.200900343. Chem Biodivers. 2010. PMID: 20232325 Review.
Cited by
-
In vitro selection of BNA (LNA) aptamers.Artif DNA PNA XNA. 2013 Apr-Jun;4(2):39-48. doi: 10.4161/adna.25786. Artif DNA PNA XNA. 2013. PMID: 24044051 Free PMC article. Review.
-
Aptamers as theranostic agents: modifications, serum stability and functionalisation.Sensors (Basel). 2013 Oct 10;13(10):13624-37. doi: 10.3390/s131013624. Sensors (Basel). 2013. PMID: 24152925 Free PMC article. Review.
-
Nucleic Acid aptamers: new methods for selection, stabilization, and application in biomedical science.Biomol Ther (Seoul). 2013 Nov;21(6):423-34. doi: 10.4062/biomolther.2013.085. Biomol Ther (Seoul). 2013. PMID: 24404332 Free PMC article. Review.
-
A New Theranostic System Based on Endoglin Aptamer Conjugated Fluorescent Silica Nanoparticles.Theranostics. 2017 Oct 17;7(19):4862-4876. doi: 10.7150/thno.19101. eCollection 2017. Theranostics. 2017. PMID: 29187909 Free PMC article.
-
Chemical synthesis of LNA-2-thiouridine and its influence on stability and selectivity of oligonucleotide binding to RNA.Biochemistry. 2009 Nov 24;48(46):10882-93. doi: 10.1021/bi901506f. Biochemistry. 2009. PMID: 19835380 Free PMC article.
References
-
- Jayasena S.D. (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin. Chem., 45, 1628–1650. - PubMed
-
- Sullenger B.A. and Gilboa,E. (2002) Emerging clinical applications of RNA. Nature, 418, 252–258. - PubMed
-
- Rimmele M. (2003) Nucleic acid aptamers as tools and drugs: recent developments. Chembiochem, 4, 963–971. - PubMed
-
- Tuerk C. and Gold,L. (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science, 249, 505–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

