Functional characterization of wild-type and mutant human sialin
- PMID: 15510212
- PMCID: PMC533050
- DOI: 10.1038/sj.emboj.7600464
Functional characterization of wild-type and mutant human sialin
Abstract
The modification of cell surface lipids or proteins with sialic acid is essential for many biological processes and several diseases are caused by defective sialic acid metabolism. Sialic acids cleaved off from degraded sialoglycoconjugates are exported from lysosomes by a membrane transporter, named sialin, which is defective in two allelic inherited diseases: infantile sialic acid storage disease (ISSD) and Salla disease. To develop a functional assay of human sialin, we redirected the protein to the plasma membrane by mutating a dileucine-based internalization motif. Cells expressing the plasmalemmal construct accumulated neuraminic acid at acidic pH by a process equivalent to lysosomal efflux. The assay was used to determine how pathogenic mutations affect transport. Interestingly, while two missense mutations and one small, in-frame deletion associated with ISSD abolished transport, the mutation causing Salla disease (R39C) slowed down, but did not stop, the transport cycle, thus explaining why the latter disorder is less severe. Since neurological symptoms predominate in Salla disease, our results suggest that sialin is rate-limiting to specific sialic acid-dependent processes of the nervous system.
Figures

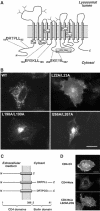
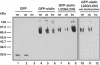


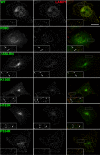
References
-
- Aula N, Jalanko A, Aula P, Peltonen L (2002) Unraveling the molecular pathogenesis of free sialic acid storage disorders: altered targeting of mutant sialin. Mol Genet Metab 77: 99–107 - PubMed
-
- Aula N, Kopra O, Jalanko A, Peltonen L (2004) Sialin expression in the CNS implicates extralysosomal function in neurons. Neurobiol Dis 15: 251–261 - PubMed
-
- Aula P, Gahl WA (2001) Disorders of free sialic acid storage. In The Metabolic and Molecular Bases of Inherited Disease, Scriver CR, Beaudet AL, Sly WS, Valle D (eds), pp 5109–5120. New York: McGraw-Hill
-
- Autio-Harmainen H, Oldfors A, Sourander P, Renlund M, Dammert K, Simila S (1988) Neuropathology of Salla disease. Acta Neuropathol (Berl) 75: 481–490 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

