Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells
- PMID: 15513935
- PMCID: PMC1665541
- DOI: 10.1113/jphysiol.2004.077289
Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells
Abstract
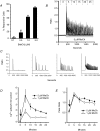
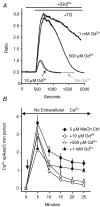
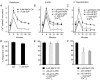
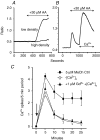
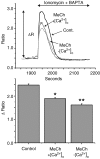
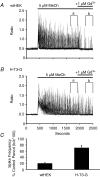
Treatment of human epithelial kidney (HEK293) cells with low concentrations of the muscarinic agonist methacholine results in the activation of complex and repetitive cycling of intracellular calcium ([Ca(2+)](i)), known as [Ca(2+)](i) oscillations. These oscillations occur with a frequency that depends on the concentration of methacholine, whereas the magnitude of the [Ca(2+)](i) spikes does not. The oscillations do not persist in the absence of extracellular Ca(2+), leading to the conclusion that entry of Ca(2+) across the plasma membrane plays a significant role in either their initiation or maintenance. However, treatment of cells with high concentrations of GdCl(3), a condition which limits the flux of calcium ions across the plasma membrane in both directions, allows sustained [Ca(2+)](i) oscillations to occur. This suggests that the mechanisms that both initiate and regenerate [Ca(2+)](i) oscillations are intrinsic to the intracellular milieu and do not require entry of extracellular Ca(2+). This would additionally suggest that, under normal conditions, the role of calcium entry is to sustain [Ca(2+)](i) oscillations. By utilizing relatively specific pharmacological manoeuvres we provide evidence that the Ca(2+) entry that supports Ca(2+) oscillations occurs through the store-operated or capacitative calcium entry pathway. However, by artificial introduction of a non-store-operated pathway into the cells (TRPC3 channels), we find that other Ca(2+) entry mechanisms can influence oscillation frequency in addition to the store-operated channels.
Figures
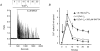

Comment in
-
What's in store for Ca2+ oscillations?J Physiol. 2005 Feb 1;562(Pt 3):645. doi: 10.1113/jphysiol.2004.078592. Epub 2004 Nov 11. J Physiol. 2005. PMID: 15539392 Free PMC article. No abstract available.
References
-
- Berridge MJ. Inositol trisphosphate and calcium oscillations. Adv Second Messenger Phosphoprotein Res. 1992;26:211–223. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bird GJS, Wedel BJ, Lièvremont J-P, Trebak M, Aziz O, Vazquez G, Putney JW., Jr Mechanisms of phospholipase C-regulated calcium entry. Current Mol Med. 2004;4:291–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

