Raised dietary n-6 polyunsaturated fatty acid intake increases 2-series prostaglandin production during labour in the ewe
- PMID: 15513945
- PMCID: PMC1665502
- DOI: 10.1113/jphysiol.2004.071969
Raised dietary n-6 polyunsaturated fatty acid intake increases 2-series prostaglandin production during labour in the ewe
Abstract
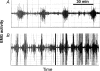
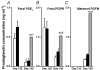
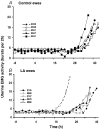
Preterm labour is the major cause of perinatal morbidity and mortality in humans. The incidence is around 10% and the causes are often unknown. Consumption of dietary n-6 polyunsaturated fatty acids (PUFAs) in western societies is increasing. These are metabolized to arachidonic acid, the precursor for 2-series prostaglandins (PGs), major signalling molecules during labour. This study investigated the effect of dietary supplementation with linoleic acid (LA, 18: 2, n-6) on parturition. Ewes were fed a control or LA-supplemented diet from 100 days gestation. Labour was induced using a standardized glucocorticoid challenge (dexamethasone, Dex) to the fetus, starting on day 139. Electromyographic (EMG) activity and fetal and maternal circulating PG concentrations were monitored. One third of LA-fed ewes delivered early (pre-Dex) although basal uterine EMG activity preceding Dex was higher in control ewes (P < 0.05). A steep increase in EMG activity occurred 18-38 h after the start of Dex infusion. Twice basal EMG activity (defined as established labour) occurred on average 7 h earlier in the LA-supplemented ewes (P < 0.05). The basal concentrations of maternal and fetal PGFM and fetal PGE(2) were approximately doubled in LA-supplemented ewes before the start of Dex infusion (P < 0.01). The rise in fetal PGE(2) and maternal oestradiol concentrations post-Dex occurred earlier in the LA-supplemented ewes. All PG measurements remained significantly higher in the LA-supplemented ewes during labour onset. This study suggests that consumption of a high LA diet in late pregnancy can enhance placental PG production and may thus increase the risk of preterm labour.
Figures


Similar articles
-
The effect of a diet supplemented with the n-6 polyunsaturated fatty acid linoleic acid on prostaglandin production in early- and late-pregnant ewes.J Endocrinol. 2005 Jan;184(1):165-78. doi: 10.1677/joe.1.05910. J Endocrinol. 2005. PMID: 15642793
-
Alteration of prostaglandin production and agonist responsiveness by n-6 polyunsaturated fatty acids in endometrial cells from late-gestation ewes.J Endocrinol. 2004 Aug;182(2):249-56. doi: 10.1677/joe.0.1820249. J Endocrinol. 2004. PMID: 15283685
-
Effects of n-6 polyunsaturated fatty acids on prostaglandin production in ovine fetal chorion cells in vitro in late gestation ewes.Placenta. 2011 Oct;32(10):752-6. doi: 10.1016/j.placenta.2011.06.026. Epub 2011 Jul 26. Placenta. 2011. PMID: 21794911
-
Nutritional regulation of uteroplacental prostaglandin production and metabolism in pregnant ewes and mares during late gestation.Exp Clin Endocrinol. 1994;102(3):212-21. doi: 10.1055/s-0029-1211285. Exp Clin Endocrinol. 1994. PMID: 7995343 Review.
-
Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour.Front Biosci. 2007 Jan 1;12:1329-43. doi: 10.2741/2151. Front Biosci. 2007. PMID: 17127385 Review.
Cited by
-
Evaluating the effect of maternal non-communicable disease on adverse pregnancy outcomes and birthweight in Pakistan, a facility based retrospective cohort study.Sci Rep. 2024 Jan 5;14(1):571. doi: 10.1038/s41598-023-51122-z. Sci Rep. 2024. PMID: 38177278 Free PMC article.
-
Autistic children exhibit decreased levels of essential Fatty acids in red blood cells.Int J Mol Sci. 2015 May 4;16(5):10061-76. doi: 10.3390/ijms160510061. Int J Mol Sci. 2015. PMID: 25946342 Free PMC article.
-
Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Effects on Growth, Metabolism, and Body Composition of the Offspring.Animals (Basel). 2020 Oct 22;10(11):1946. doi: 10.3390/ani10111946. Animals (Basel). 2020. PMID: 33105758 Free PMC article.
-
Maternal obesity-induced decreases in plasma, hepatic and uterine polyunsaturated fatty acids during labour is reversed through improved nutrition at conception.Sci Rep. 2018 Feb 21;8(1):3389. doi: 10.1038/s41598-018-21809-9. Sci Rep. 2018. PMID: 29467407 Free PMC article.
References
-
- Abel-Caines SF, Grant RJ, Klopfenstein TJ, Winowski T, Barney N. Influence of non enzymatically browned soybeans on rumenal fermentation and lactational performance of dairy cows. J Dairy Sci. 1998;81:1036–1045. - PubMed
-
- Allen KGD, Harris MA. The role of n-3 fatty acids in gestation and parturition. Exp Biol Med. 2001;226:498–506. - PubMed
-
- Araya J, Rojas M, Fernandez P, Mateluna A. Essential fatty acid content of maternal erythrocyte phospholipids. A study in preterm and full term human newborns. Rev Med Chil. 1998;126:391–396. - PubMed
-
- Baguma-Nibasheka M, Brenna JT, Nathanielsz PW. Delay of pre-term delivery in sheep by omega-3 long chain polyunsaturates. Biol Reprod. 1999;60:698–701. - PubMed
-
- Challis JRG, Lye SJ, Gibb W. Prostaglandins and parturition. Ann NY Acad Sci. 1997;26:247–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

