Oxetane modified, conformationally constrained, antisense oligodeoxyribonucleotides function efficiently as gene silencing molecules
- PMID: 15514112
- PMCID: PMC528787
- DOI: 10.1093/nar/gkh893
Oxetane modified, conformationally constrained, antisense oligodeoxyribonucleotides function efficiently as gene silencing molecules
Abstract
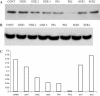
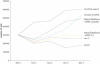
Incorporation of nucleosides with novel base-constraining oxetane (OXE) modifications [oxetane, 1-(1',3'-O-anhydro-beta-d-psicofuranosyl nucleosides)] into antisense (AS) oligodeoxyribonucleotides (ODNs) should greatly improve the gene silencing efficiency of these molecules. This is because OXE modified bases provide nuclease protection to the natural backbone ODNs, can impart T(m) values similar to those predicted for RNA-RNA hybrids, and not only permit but also accelerate RNase H mediated catalytic activity. We tested this assumption in living cells by directly comparing the ability of OXE and phosphorothioate (PS) ODNs to target c-myb gene expression. The ODNs were targeted to two different sites within the c-myb mRNA. One site was chosen arbitrarily. The other was a 'rational' choice based on predicted hybridization accessibility after physical mapping with self-quenching reporter molecules (SQRM). The Myb mRNA and protein levels were equally diminished by OXE and PS ODNs, but the latter were delivered to cells with approximately six times greater efficiency, suggesting that OXE modified ODNs were more potent on a molar basis. The rationally targeted molecules demonstrated greater silencing efficiency than those directed to an arbitrarily chosen mRNA sequence. We conclude that rationally targeted, OXE modified ODNs, can function efficiently as gene silencing agents, and hypothesize that they will prove useful for therapeutic purposes.
Figures
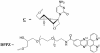

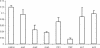

References
-
- Dykxhoorn D.M., Novina,C.D. and Sharp,P.A. (2003) Killing the messenger: short RNAs that silence gene expression. Nature Rev. Mol. Cell. Biol., 4, 457–467. - PubMed
-
- Jansen B. and Zangemeister-Wittke,U. (2002) Antisense therapy for cancer—the time of truth. Lancet Oncol., 3, 672–683. - PubMed
-
- Kurreck J. (2003) Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem., 270, 1628–1644. - PubMed
-
- Opalinska J.B. and Gewirtz,A.M. (2002) Nucleic-acid therapeutics: basic principles and recent applications. Nature Rev. Drug Discov., 1, 503–514. - PubMed
-
- Matteucci M.D. and Wagner,R.W. (1996) In pursuit of antisense. Nature, 384, 20–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

