Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial
- PMID: 15514344
- PMCID: PMC524548
- DOI: 10.1136/bmj.329.7473.1004
Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial
Abstract
Objective: To test a multifaceted collaborative quality improvement intervention designed to promote evidence based surfactant treatment for preterm infants of 23-29 weeks' gestation.
Design: Cluster randomised controlled trial.
Setting and participants: 114 neonatal intensive care units (which treated 6039 infants of 23-29 weeks gestation born in 2001).
Main outcome measures: Process of care measures: proportion of infants receiving first surfactant in the delivery room, proportion receiving first surfactant more than two hours after birth, and median time from birth to first dose of surfactant. Clinical outcomes: death before discharge home, and pneumothorax.
Intervention: Multifaceted collaborative quality improvement advice including audit and feedback, evidence reviews, an interactive training workshop, and ongoing faculty support via conference calls and email.
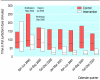
Results: Compared with those in control hospitals, infants in intervention hospitals were more likely to receive surfactant in the delivery room (adjusted odds ratio 5.38 (95% confidence interval 2.84 to 10.20)), were less likely to receive the first dose more than two hours after birth (adjusted odds ratio 0.35 (0.24 to 0.53)), and received the first dose of surfactant sooner after birth (median of 21 minutes v 78 minutes, P < 0.001). The intervention effect on timing of surfactant was larger for infants born in the participating hospitals than for infants transferred to a participating hospital after birth. There were no significant differences in mortality or pneumothorax.
Conclusion: A multifaceted intervention including audit and feedback, evidence reviews, quality improvement training, and follow up support changed the behaviour of health professionals and promoted evidence based practice.
Figures

Comment in
-
A collaborative quality improvement intervention was effective for promoting use of surfactant therapy in preterm infants.Evid Based Nurs. 2005 Jul;8(3):90. doi: 10.1136/ebn.8.3.90. Evid Based Nurs. 2005. PMID: 16021721 No abstract available.
References
-
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington DC: National Academy Press, 2001.
-
- Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2001;(2): CD000510. - PubMed
-
- Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2000;(2): CD001456. - PubMed
-
- Horbar JD, Carpenter J, Buzas J, Soll RF, Suresh G, Bracken MB, et al. Timing of initial surfactant treatment for infants 23 to 29 weeks gestation: is routine practice evidence based? Pediatrics. 2004;113: 1593-602. - PubMed
-
- Jamtvedt G, Young JM, Kristoffersen DT, Thomson O'Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2003;(3): CD000259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
