Pax6 guides a relay of pioneer longitudinal axons in the embryonic mouse forebrain
- PMID: 15514979
- PMCID: PMC2080865
- DOI: 10.1002/cne.20317
Pax6 guides a relay of pioneer longitudinal axons in the embryonic mouse forebrain
Abstract
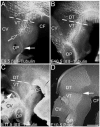
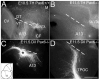
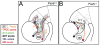
We have characterized a system of early neurons that establish the first two major longitudinal tracts in the embryonic mouse forebrain. Axon tracers and antibody labels were used to map the axon projections in the thalamus from embryonic days 9.0-12, revealing several distinct neuron populations that contributed to the first tracts. Each of the early axon populations first grew independently, pioneering a short segment of new tract. However, each axon population soon merged with other axons to form one of only two shared longitudinal tracts, both descending: the tract of the postoptic commissure (TPOC), and, in parallel, the stria medullaris. Thus, the forebrain longitudinal tracts are pioneered by a relay of axons, with distinct axon populations pioneering successive segments of these pathways. The extensive merging of tracts suggests that axon-axon interactions are a major guidance mechanism for longitudinal axons. Several axon populations express tyrosine hydroxylase, identifying the TPOC as a major pathway for forebrain dopaminergic projections. To start a genetic analysis of pioneer axon guidance, we have identified the transcription factor Pax6 as critical for tract formation. In Pax6 mutants, both longitudinal tracts failed to form due to errors by every population of early longitudinal axons. Taken together, these results have identified potentially important interactions between series of pioneer axons and the Pax6 gene as a general regulator of longitudinal tract formation in the forebrain.
Figures



References
-
- Andrews GL, Yun K, Rubenstein JL, Mastick GS. Dlx transcription factors regulate differentiation of dopaminergic neurons of the ventral thalamus. Mol Cell Neurosci. 2003;23:107–120. - PubMed
-
- Auladell C, Perez-Sust P, Super H, Soriano E. The early development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol (Berl) 2000;201:169–179. - PubMed
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

