Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense
- PMID: 15516507
- PMCID: PMC527161
- DOI: 10.1104/pp.104.048900
Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense
Abstract
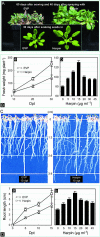
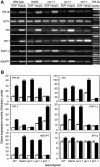
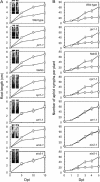
Ethylene (ET) signal transduction may regulate plant growth and defense, depending on which components are recruited into the pathway in response to different stimuli. We report here that the ET pathway controls both insect resistance (IR) and plant growth enhancement (PGE) in Arabidopsis (Arabidopsis thaliana) plants responding to harpin, a protein produced by a plant pathogenic bacterium. PGE may result from spraying plant tops with harpin or by soaking seeds in harpin solution; the latter especially enhances root growth. Plants treated similarly develop resistance to the green peach aphid (Myzus persicae). The salicylic acid pathway, although activated by harpin, does not lead to PGE and IR. By contrast, PGE and IR are induced in both wild-type plants and genotypes that have defects in salicylic acid signaling. In response to harpin, levels of jasmonic acid (JA) decrease, and the COI1 gene, which is indispensable for JA signal transduction, is not expressed in wild-type plants. However, PGE and IR are stimulated in the JA-resistant mutant jar1-1. In the wild type, PGE and IR develop coincidently with increases in ET levels and the expression of several genes essential for ET signaling. The ET receptor gene ETR1 is required because both phenotypes are arrested in the etr1-1 mutant. Consistently, inhibition of ET perception nullifies the induction of both PGE and IR. The signal transducer EIN2 is required for IR, and EIN5 is required for PGE because IR and PGE are impaired correspondingly in the ein2-1 and ein5-1 mutants. Therefore, harpin activates ET signaling while conscribing EIN2 and EIN5 to confer IR and PGE, respectively.
Figures



References
-
- Alonso JM, Hirayama T, Roman G, Nourizadehm S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 - PubMed
-
- Andi S, Taguchi F, Toyoda K, Shiraishi T, Ichinose Y (2001) Effect of methyl jasmonate on harpin-induced hypersensitive cell death, generation of hydrogen peroxide and expression of PAL mRNA in tobacco suspension cultured BY-2 cells Plant Cell Physiol 42: 446–449 - PubMed
-
- Bauer DW, Wei ZM, Beer SV, Collmer A (1995) Erwinia chrysanthemi harpinEch: an elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol Plant Microbe Interact 8: 484–491 - PubMed
-
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 - PubMed
-
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

