Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase
- PMID: 15516513
- PMCID: PMC527178
- DOI: 10.1104/pp.104.044503
Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase
Abstract
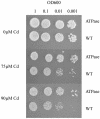
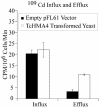
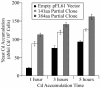
Thlaspi caerulescens is a heavy metal hyperaccumulator plant species that is able to accumulate extremely high levels of zinc (Zn) and cadmium (Cd) in its shoots (30,000 microg g(-1) Zn and 10,000 microg g(-1) Cd), and has been the subject of intense research as a model plant to gain a better understanding of the mechanisms of heavy metal hyperaccumulation and tolerance and as a source of genes for developing plant species better suited for the phytoremediation of metal-contaminated soils. In this study, we report on the results of a yeast (Saccharomyces cerevisae) complementation screen aimed at identifying candidate heavy metal tolerance genes in T. caerulescens. A number of Thlaspi genes that conferred Cd tolerance to yeast were identified, including possible metal-binding ligands from the metallothionein gene family, and a P-type ATPase that is a member of the P1B subfamily of purported heavy metal-translocating ATPases. A detailed characterization of the Thlaspi heavy metal ATPase, TcHMA4, demonstrated that it mediates yeast metal tolerance via active efflux of a number of different heavy metals (Cd, Zn, lead [Pb], and copper [Cu]) out of the cell. However, in T. caerulescens, based on differences in tissue-specific and metal-responsive expression of this transporter compared with its homolog in Arabidopsis (Arabidopsis thaliana), we suggest that it may not be involved in metal tolerance. Instead, we hypothesize that it may play a role in xylem loading of metals and thus could be a key player in the hyperaccumulation phenotype expressed in T. caerulescens. Additionally, evidence is presented showing that the C terminus of the TcHMA4 protein, which contains numerous possible heavy metal-binding His and Cys repeats residues, participates in heavy metal binding. When partial peptides from this C-terminal domain were expressed in yeast, they conferred an extremely high level of Cd tolerance and Cd hyperaccumulation. The possibilities for enhancing the metal tolerance and phytoremediation potential of higher plants via expression of these metal-binding peptides are also discussed.
Figures





References
-
- Axelsen KB, Palmgren MG (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46: 84–101 - PubMed
-
- Brooks RR, Lee J, Reeves RD, Jaffre T (1977) Detection of metalliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor 7: 49–77
-
- Brown S, Chaney R, Angle J, Baker A (1995) Zinc and cadmium uptake of T. caerulescens grown in nutrient solution. Soil Sci Soc Am J 59: 125–133
-
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 457–486 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

