Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies
- PMID: 15516516
- PMCID: PMC527176
- DOI: 10.1104/pp.104.042036
Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies
Abstract
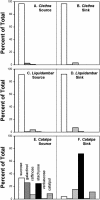
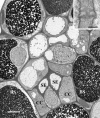
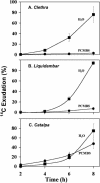
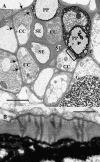
The incidence of plasmodesmata in the minor vein phloem of leaves varies widely between species. On this basis, two pathways of phloem loading have been proposed: symplastic where frequencies are high, and apoplastic where they are low. However, putative symplastic-loading species fall into at least two categories. In one, the plants translocate raffinose-family oligosaccharides (RFOs). In the other, the primary sugar in the phloem sap is sucrose (Suc). While a thermodynamically feasible mechanism of symplastic loading has been postulated for species that transport RFOs, no such mechanism is known for Suc transporters. We used p-chloromercuribenzenesulfonic acid inhibition of apoplastic loading to distinguish between the two pathways in three species that have abundant minor vein plasmodesmata and are therefore putative symplastic loaders. Clethra barbinervis and Liquidambar styraciflua transport Suc, while Catalpa speciosa transports RFOs. The results indicate that, contrary to the hypothesis that all species with abundant minor vein plasmodesmata load symplastically, C. barbinervis and L. styraciflua load from the apoplast. C. speciosa, being an RFO transporter, loads from the symplast, as expected. Data from these three species, and from the literature, also indicate that plants with abundant plasmodesmata in the minor vein phloem have abundant plasmodesmata between mesophyll cells. Thus, plasmodesmatal frequencies in the minor veins may be a reflection of overall frequencies in the lamina and may have limited relevance to phloem loading. We suggest that symplastic loading is restricted to plants that translocate oligosaccharides larger than Suc, such as RFOs, and that other plants, no matter how many plasmodesmata they have in the minor vein phloem, load via the apoplast.
Figures


References
-
- Beebe DU, Evert RF (1992) Photoassimilate pathways and phloem loading in the leaf of Moricandia arvensis L. Dc. Brassicaceae. Int J Plant Sci 153: 61–77
-
- Beebe DU, Russin WA (1999) Plasmodesmata in the phloem-loading pathway. In AJE van Bel, WJP Van Kesteren, eds, Plasmodesmata: Structure, Function, Role in Cell Communication. Springer-Verlag, New York, pp 261–293
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

