Responses of the Rhodobacter sphaeroides transcriptome to blue light under semiaerobic conditions
- PMID: 15516587
- PMCID: PMC524910
- DOI: 10.1128/JB.186.22.7726-7735.2004
Responses of the Rhodobacter sphaeroides transcriptome to blue light under semiaerobic conditions
Abstract
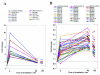
Exposure to blue light of the facultative phototrophic proteobacterium Rhodobacter sphaeroides grown semiaerobically results in repression of the puc and puf operons involved in photosystem formation. To reveal the genome-wide effects of blue light on gene expression and the underlying photosensory mechanisms, transcriptome profiles of R. sphaeroides during blue-light irradiation (for 5 to 135 min) were analyzed. Expression of most photosystem genes was repressed upon irradiation. Downregulation of photosystem development may be used to prevent photooxidative damage occurring when the photosystem, oxygen, and high-intensity light are present simultaneously. The photoreceptor of the BLUF-domain family, AppA, which belongs to the AppA-PpsR antirepressor-repressor system, is essential for maintenance of repression upon prolonged irradiation (S. Braatsch et al., Mol. Microbiol. 45:827-836, 2002). Transcriptome data suggest that the onset of repression is also mediated by the AppA-PpsR system, albeit via an apparently different sensory mechanism. Expression of several genes, whose products may participate in photooxidative damage defense, including deoxypyrimidine photolyase, glutathione peroxidase, and quinol oxidoreductases, was increased. Among the genes upregulated were genes encoding two sigma factors: sigmaE and sigma38. The consensus promoter sequences for these sigma factors were predicted in the upstream sequences of numerous upregulated genes, suggesting that coordinated action of sigmaE and sigma38 is responsible for the upregulation. Based on the dynamics of upregulation, the anti-sigmaE factor ChrR or its putative upstream partner is proposed to be the primary sensor. The identified transcriptome responses provided a framework for deciphering blue-light-dependent signal transduction pathways in R. sphaeroides.
Figures

References
-
- Ahmad, M., and A. R. Cashmore. 1993. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162-166. - PubMed
-
- Baca, M., G. E. O. Borgstahl, M. Boissinot, P. M. Burke, D. R. Williams, K. A. Slater, and E. D. Getzoff. 1994. Complete chemical structure of photoactive yellow protein: novel thioester-linked 4-hydroxy-cinnamyl chromophore and photocycle chemistry. Biochemistry 33:14369-14377. - PubMed
-
- Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. - PubMed
-
- Berrocal-Tito, G., L. Sametz-Baron, K. Eichenberg, B. A. Horwitz, and A. Herrera-Estrella. 1999. Rapid blue light regulation of a Trichoderma harzianum photolyase gene. J. Biol. Chem. 274:14288-14294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

