Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells
- PMID: 15520245
- PMCID: PMC2211867
- DOI: 10.1084/jem.20041408
Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells
Abstract
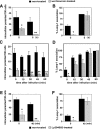
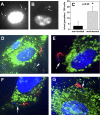
Trypomastigotes, the highly motile infective forms of Trypanosoma cruzi, are capable of infecting several cell types. Invasion occurs either by direct recruitment and fusion of lysosomes at the plasma membrane, or through invagination of the plasma membrane followed by intracellular fusion with lysosomes. The lysosome-like parasitophorous vacuole is subsequently disrupted, releasing the parasites for replication in the cytosol. The role of this early residence within lysosomes in the intracellular cycle of T. cruzi has remained unclear. For several other cytosolic pathogens, survival inside host cells depends on an early escape from phagosomes before lysosomal fusion. Here, we show that when lysosome-mediated T. cruzi invasion is blocked through phosophoinositide 3-kinase inhibition, a significant fraction of the internalized parasites are not subsequently retained inside host cells for a productive infection. A direct correlation was observed between the lysosomal fusion rates after invasion and the intracellular retention of trypomastigotes. Thus, formation of a parasitophorous vacuole with lysosomal properties is essential for preventing these highly motile parasites from exiting host cells and for allowing completion of the intracellular life cycle.
Figures




References
-
- Chagas, C. 1909. Nova trypanozomiaze humana. Mem. Inst. Oswaldo Cruz. 1:11–80.
-
- Brener, Z. 1973. Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27:347–383. - PubMed
-
- Tardieux, I., P. Webster, J. Ravesloot, W. Boron, J.A. Lunn, J.E. Heuser, and N.W. Andrews. 1992. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 71:1117–1130. - PubMed

