The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity
- PMID: 15520246
- PMCID: PMC2211858
- DOI: 10.1084/jem.20041185
The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity
Abstract
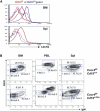
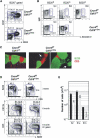
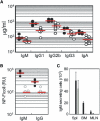
The chemokine receptor CXCR4 is expressed in B cells at multiple stages of their development. CXCR4 function in humoral immunity has not been fully investigated. We have generated gene-targeted mice in which CXCR4 can be selectively inactivated in B cells and have shown that it is required for retention of B cell precursors in the bone marrow. CXCR4-deficient B cell precursors that migrated prematurely became localized in splenic follicles despite their unresponsiveness to CXCL13. Concomitantly, mature B cell populations were reduced in the splenic marginal zone and primary follicles, and in the peritoneal cavity in the mutant animals, as were T-independent antibody responses. In addition, aberrant B cell follicles formed ectopically in intestinal lamina propria around Peyer's patches. These findings establish an important role for CXCR4 in regulating homeostasis of B cell compartmentalization and humoral immunity.
Figures



References
-
- Carsetti, R., M.M. Rosado, and H. Wardmann. 2004. Peripheral development of B cells in mouse and man. Immunol. Rev. 197:179–191. - PubMed
-
- Tanigaki, K., H. Han, N. Yamamoto, K. Tashiro, M. Ikegawa, K. Kuroda, A. Suzuki, T. Nakano, and T. Honjo. 2002. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 3:443–450. - PubMed
-
- Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. - PubMed
-
- Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. - PubMed
-
- Cyster, J.G., K.M. Ansel, K. Reif, E.H. Ekland, P.L. Hyman, H.L. Tang, S.A. Luther, and V.N. Ngo. 2000. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176:181–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

